Journal of Cancer Research and Immuno-Oncology
Open Access
ISSN: 2684-1266
ISSN: 2684-1266
Research Article - (2023)Volume 9, Issue 4
Single brain metastasis surgical resection remains an effective treatment for brain metastases. However, surgery alone associate with a high rate of local failure. The current guidelines recommend radiation to surgical cavity. The recommended dose for brain cavity after surgery remain to be define as most guidelines gives a very general suggestion about the dose and fractionation. The aim of this study was to determine what variable influence local control in this specific population. Methods Retrospective analysis of 52 patients with breast cancer who undergo surgical resection to single brain metastases and who received post-operative radiotherapy between the years 2010- 2022. All clinical and dosimetric variables were analyzed to evaluate their impact on local control. A predictive model for local control was calculated. Results One year local control was 65.3%. HER-2 disease, size of initial tumor, time from initial surgery, total dose deliver and the type of lesion were all significant for local control. The most important variable was total dose deliver. A specific nomogram using 7 parameters for a prediction of local was computed. Conclusion Brain metastases from breast cancer remain a life-threating condition. Surgery plays a critical role in the treatment of large symptomatic brain metastases for which Post-operative radiotherapy is essential. Choosing a regime of 5 fraction with a dose of 30 Gy (BED4>70 Gy) seem important for achieving local control and without increase toxicity.
Breast cancer; Brain metastases; Post resection radiotherapy; Fractionated radio-surgery
Bain metastases are a major cause of mortality in breast cancer [1]. The brain is the first site of metastasis from breast cancer in 12% of patients. It has been suggested that brain metastases from Breast Cancer (BMBC) occur more frequently among younger women, those with larger tumors or higher nuclear grade, in certain subtypes such as Estrogen-Receptor (ER)-negative and HER2 overexpressing tumors, and those with nodal metastases [2].
Single brain metastasis surgical resection remains an effective treatment for brain metastases, especially for larger lesions causing mass effect and consequentially serious neurological symptoms [3]. However, surgery alone associate with a very high rate of local that can be high up to 70% for 1 year [4,5].
Data from two randomized trials have demonstrated that SRS to the resection cavity significantly reduces bed recurrence rates compared with observation alone and decreases the risk of cognitive decline in patients with brain metastases as compared to Whole brain radiation, without diminishing survival [6,7].
The current guidelines of both Society of neuro-oncology and the international stereotactic radiosurgery recommend radiation to surgical cavity [8]. The recommended dose for brain cavity after surgery remain to be define as most guidelines gives a very general suggestion about the dose and fractionation. In addition, specific dose control relationship has never been published for breast cancer metastases. The unique radio-sensitivity and other biological aspect of this etiology need to be addressed.
Here we perform a comprehensive analysis of dosimetry, biology and clinical variable and their influence on local control and on brain failure among breast cancer patients who been treated with surgery and post-operative fractionated stereotactic radiotherapy for a single brain metastasis.
Institutional review board approval for a retrospective review was obtained. Inclusion criteria were all patients treated between 2013-2022 with radiotherapy to the surgical cavity after craniotomy for a single brain metastases of breast cancer origin based upon pathology report. We excluded patients who had previously received either RT or WBRT or neo-adjuvant SRS for the resected lesion. We analyzed only patients who had 1 year follow up.
Background demographics, pathologic and radiographic data, prior oncologic therapy, and detailed radiotherapy data was extracted from the electronic medical record and from institutional radiotherapy databases. Local failure was defined as tumor growth in the surgical cavity inside the Planning target volume as defined by other works [7]. Time to failure was calculated from the completion of radiotherapy. Toxicity was evaluated and graded by CTCAE v5.0 criteria.
Statistical analysis
Descriptive analyses were performed using mean and SD for parametric variables and median with range for non-parametric variables. X2 test was used for categorical variables. Time-to event outcomes were estimated using the Kaplan–Meier method. Total dose (BED) and dose per fraction were analyzed as both a continues and categorical variables at different thresholds. A Cox regression model was applied to study variables shown to have an impact on local control Data was analyzed using statistical software SPSS V26 (version 26, IBM©, Armonk, NY, USA). After evaluation of different cutoff of different variable we created the best fit model of logistic regression model and compute from that a nomogram for prediction of local control using several parameters.
A total of 52 patients with the diagnosis of brain metastatic breast cancer who had undergone surgery and post-operative radiation were treated at our institution from 2010-2022. The median age was 45 years. Breast cancer type was predominantly HER-2 positive (42.3%), among them two were ER/PR positive and six were ER/PR negative. 36% were ER/PR positive, HER-2 negative and 21% were triple negative.
Patient's presentation was variable. The most frequent complaint was loss of gross limb weakness with secondary headache, aphasia and imbalance. 38.4% of patients had widespread systemic metastatic disease while surgery. Only two patients had solitary brain lesion.
The most frequent location was frontal lobe (38.4%). Solid appearance was more prevalent than cystic (73.1% and 26.9% respectively). Radiation was initiated on average 37 days from surgery with a range of 24 up to 61 days. Patients' characteristics are presented in Table 1.
| Variable | |
| Number of patients | 52 |
| Age (mean, range) | 45 (23-68) |
| Breast CA type | |
| ER+/PR+, Her-2- ER+/PR+,HER-2+ ER-/PR-,HER-2+ Triple negative |
16 8 16 12 |
| GPA | |
| 0-1 1.5-2 2.5-3 3.5-4 |
0 8 20 24 |
| Size of metastases | |
| 0.5-1.5 cm 1.5-2.5 cm 2.5-3.5 cm >3.5 cm |
8 12 14 18 |
| Location | |
| Frontal Parietal Temporal Occipital Cerebellum |
20 10 8 6 8 |
| Type of lesion | |
| Solid Cystic |
38 14 |
| Time from surgery to RT in Days (mean, range) | 33 (20-61) |
| Systemic disease status after resection | |
| Solitary brain lesion Oligo-metastases (<5 mets) extensive metastatic disease |
10 8 20 |
Table 1: Patients characteristics.
Radiation treatment
A total of 30 radiation treatment were delivered using VMAT, 20 using IMRT and two with 3D planning. A BED calculated using α/β of 10 (common cancer reference) and of 4 (breast cancer). Using this approach, the median dose was 37.5 Gy (28 Gy to 57.6 Gy(BED10)) and 56.25 Gy (40Gy-96 GY(BED4)) respectively.
In 24 cases the tumor was with contact of dura. Six were also in contact with the venous sinus. Clinical Target Volume (CTV) was defined as the surgical cavity. CTV to Planning Target Volume (PTV) was expand 2-5 mm. In 61.5% of cases the surgical corridor was included in the CTV. During this analysis we used the TG101 report constrains [8]. In all 52 treatment planning dose constrain were met. Radiotherapy parameters are shown in Table 2.
| Variable | |
|---|---|
| PTV (median, range) | 67.1 cc (23.4-112.6) |
| Dose (BED α/β=10) (median, range) | 37.5 Gy (28-57.6) |
| Dose (BED α/β=4) (median, range) | 56.25 Gy (40-96) |
|
Number of fraction (Median, range) |
5 (3-7) 2 8 36 4 2 |
| Brain V25 (Brain-PTV) (median, range) | 4.4 cc (0-9.1 cc) |
Table 2: Radiation parameters.
Local control
The one-year local control rate was 65.3%. Among the 18 patients that had local failure 12 received radiosurgery and 6 received systemic therapy. In 28 cases there was a distant brain failure, among these eight had leptomeningeal spread of whom six received whole brain radiotherapy and two received systemic therapy. In all other 20 cases radiosurgery was performed. Clinical and survival outcomes are presented in Table 3.
| Follow up months (median, range) | 28 (14-43) |
| 1 year LC (%) | 65.3% (17/26) |
| Radiation necrosis (%) | 15.3%(4/26) |
| Asymptomatic Symptomatic |
8 0 |
| 1 year distant brain failure (%) | 28/52 (53.8%) |
| 1 years local and distant brain failure (%) | 10/52 (19.2%) |
| 1 year overall survival (%) | 76.9% (40/52) |
Table 3: Outcome parameters.
Impact on local control
One year local control was 65.3%. We compare different variables between patients who achieved local control and those who did not. The median planning target volume was significantly larger among those who had experienced local failure (83.7 cc vs. 56.2 cc, P=0.042). In addition, the total dose delivered was lower (37.5 Gy vs. 42.6 Gy, P=0.048).
In regards to the biology of breast cancer. We notice different distribution of HER-2 positive disease among those who had local control and those who did not (47% vs. 33%, P=0.039).
There was no association between age and the location of the tumor to local control. Local control was associated with the inclusion of surgical corridor within the PTV (P=0.061) also shorter time to radiotherapy from initial surgery (p=0.059). Cystic lesion recurred locally more frequently than solid lesions (66.6% vs. 5.8%, P<0.001) (Table 4). On multivariable analysis of predictors of local control using cox regression. HER-2 positive disease, less than 37 days from surgery, solid lesion and dose above 70 Gy (BED4) and size of initial tumor less than 3.5 cm were all significant for local control (Table 5).
| Variables | Local control (n=17) | Local progression | P |
|---|---|---|---|
| (n=9) | |||
| Mean age | 45.5 Y | 44 Y | 0.87 |
| HER-2 positive% | 47% | 33% | 0.039 |
| PTV median |
55.2 cc | 83.7 cc | 0.042 |
| Size of initial tumor (median) | 2.2 cm | 3.1 cm | 0.032 |
| Dose BED (α/β=4) median |
65.3 Gy | 56.25 Gy | 0.048 |
| Inclusion of surgical corridor% | 64.70% | 55.50% | 0.061 |
| Time from surgery to radiation (days) | 30.5 days | 42.5 days | 0.056 |
| Type of metastases (Cystic) | 5.80% | 66.60% | <0.001 |
| Location in cerebellum | 17.60% | 11.10% | 0.71 |
Table 4: Local control vs. local failure.
| Variable | HR (CI), P |
|---|---|
| Age>40 Y | 0.75 (0.42-2.2), p=0.73 |
| HER-2 positive | 0.63 (0.55-0.87), p=0.037 |
| Size of initial tumor>3.5 CM | 1.61 (1.11-3.1), p=0.007 |
| Time from surgery >30 days | 1.46 (1.13-2.78), p=0.044 |
| Dose>70 Gy (BED4) | 0.51 (0.16-0.91), p=0.024 |
| Location of tumor (cerebellum vs. supratentorial) |
1.1 (0.12-3.5), p=0.41 |
| Corridor inclusion (yes) | 0.91 (0.54-2.12), p=0.53 |
| Type of metastases (Cystic vs. solid) | 1.55 (1.13-2.34), p=0.031 |
Table 5: Multivariable analysis of local failure.
In this retrospective cohort we evaluate the variable impact local control among a specific group of metastatic breast cancer undergoing surgery and post-operative RT [9,10]. We found that the tumor biology, size, and are all significant for local control in addition to time from initial surgery.
Our local control rate is lower than previously studies that showed local control rate of 70-95% [4,7,11,12]. That can be partially explained by a high percentage of patients receiving a lower dose of our trial compare to the others. In our study 46% received a BED (10) lower then 40 (i.e. 25 Gy in 5 fraction) which recently been shown by Minnti et al. to decrease local control [12].
We demonstrated correlation between dose and local control. In our analysis we used an α/β ratio of 4 Gy which is more realistic in breast cancer tumors [13]. We notice that Dose above 70 Gy (BED4) had HR of 0.51 (CI 0.16-0.91) even when adjusting to other variables.
Another explanation of the lower local rate results in our cohort is the timing from initial surgery until start of radiation. In a recent Meta-analysis of post-operative SRS showed a lower local control when surgery-to-SRS delay longer than 3 weeks. The estimated 12-month control rates dropped from 87% to 61% if SRS was performed more than 3 weeks after resection [14]. In our study the median day for starting radiation was 33. We found that starting radiation more than 30 days from surgery has a HR of 1.46 (1.13-2.78) for local failure. In regards to other parameters. The average size of tumor in our cohort was similar to other studies [15,16] and reflects the current change in practice to operate only on symptomatic large lesions, with the remainder undergoing definitive radiosurgery. Tumor larger than 3.5 cm had significantly worsen local failure with HR of 1.61 (CI 1.11-1.31).
The current guidelines recommend the inclusion of surgical tract with 1-5 mm margin [13]. In our cohort inclusion of surgical tract was seen in 64.7% of those who achieved local control and 55.5% at those who didn’t. However, this difference did not reach statistical significance.
Different studies had shown contradictory results on the impact of cystic lesions and response to local treatment [17]. Studies have suggested that the causes of cystic masses may include the breakdown of the blood-brain barrier or the higher risk of developing cystic BM in patients with poor histological grade, [18,19]. In addition, the complications seen in operations on cystic lesion and the less than gross tumor resection achieved can have an impact on overall survival and local control respectively [20]. In our study cystic had much higher risk of local failure with HR of 1.55 (1.13-2.34).
Breast cancer biology
Different classical sub types of breast cancer have different biology in regards of brain metastases prevalence, pathophysiology and response to treatment [17]. HER2-positive breast cancer has the inherent tendency of metastasis to the brain but because of variable systemic treatment options with good brain response and even longer survival among all breast cancer population with brain metastases [21].
Surprisingly, in our cohort patients the prevalence of HER-2 sub type was higher among those who achieved local control. Having HER-2 disease decrease the odds for local failure by 37% even when adjusting to different dose. This can be explained by the fact that most patients in our cohort had visceral metastatic disease and received systemic therapy after surprisingly, in our cohort patients the prevalence of HER-2 sub type was higher among those who achieved local control. Having HER-2 disease decrease the odds for local failure by 37% even when adjusting to different dose. This can be explained by the fact that most patients in our cohort had visceral metastatic disease and received systemic therapy after the course of radiation. HER-2 targeted therapy like transtuzumab, transtuzumab-emtansine, fam- transtuzumab-deruxtecan, lapatinib with capecitabine and tucatinib have all high response rate in the CNS [21] and can help reduced the risk of recurrence by effectively treating microscopic disease. This advantage is lacking in other sub-type populations. Using the cox regression analysis, we built a nomogram for local failure (Figure 1).
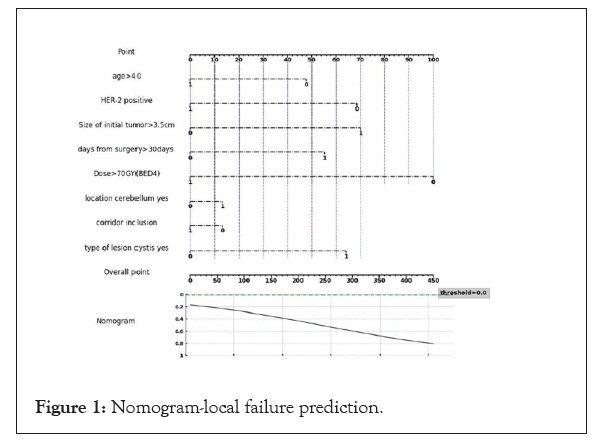
Figure 1: Nomogram-local failure prediction.
Toxicity
We found 8 cases of reported radiation necrosis on MRI. All of whom were asymptomatic. Of these patients, four received 65.31 Gy, two received 87.75 Gy and two received 72 Gy (all using BED4). 38% reported grade 2 fatigue and 11% with grade 2 headache.
Brain metastases from breast cancer remain a life-threating condition. Surgery plays a critical role in the treatment of large symptomatic brain metastases for whom post-operative radiotherapy is essential. Choosing for example a regime of 5 fraction with a dose of 30 Gy (BED4>70 Gy) seem important for achieving local control and without increase toxicity.
We have defined the clinical characteristics associated with local failure amongst brain metastases of breast cancer origin following surgical resection and post-operative irradiation. Higher radiation dose is associated with both higher rates of local control but also increased rates of radiation brain injury. Our results, that need to be confirmed in a larger study, suggest that a nomogram can help to produce an individualized dosing schedule, based upon a cost-benefit approach may be plausible and appropriate.
Citation: Feng J, Saechs G, Patel R, Liang P, Guieu N. (2023) Prediction of Local Failure for Post-Operative Radiotherapy of Resected Brain Metastases in Breast Cancer Patients. J Cancer Res Immunooncol. 09:187.
Received: 27-Nov-2023, Manuscript No. JCRIO-23-28187; Editor assigned: 29-Nov-2023, Pre QC No. JCRIO-23-28187 (PQ); Reviewed: 13-Dec-2023, QC No. JCRIO-23-28187; Revised: 20-Dec-2023, Manuscript No. JCRIO-23-28187 (R); Published: 27-Dec-2023 , DOI: 10.35248/2684-1266.23.09.197
Copyright: © 2023 Feng J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.