Research Article - (2025)Volume 10, Issue 1
Background: Metastatic prostate cancer remains one of the leading causes of cancer-related morbidity and mortality in men worldwide. Despite advances in Androgen Deprivation Therapies (ADT), chemotherapy, targeted agents, and immunotherapies, many patients develop therapeutic resistance and experience significant toxicities. Dysregulated metabolic and inflammatory pathways, such as heightened glycolysis, increased proinflammatory cytokines, elevated Matrix Metalloproteinases (particularly MMP-9), and Androgen Receptor splice variants (e.g., AR-V7), can further drive disease progression. Emerging evidence suggests that optimizing host biology through circadian rhythm support, antioxidant defense, and modulation of immune function may confer therapeutic benefits. We conducted a double-blind, placebo-controlled study in 52 men to evaluate the effects of AminoTriComplex, a natural multi-component formulation that integrates advanced “molecular activation” and liposomalization technology to enhance bioavailability. AminoTriComplex contains key plant-derived bioactive molecules and melatonin in a specialized matrix hypothesized to modulate metabolic, inflammatory, and androgen-driven processes.
Methods: Twenty-seven men with Metastatic Prostate Cancer (mPCa) composed the treatment group, while 25 agematched healthy men served as controls. The treatment group received AminoTriComplex plus standard-of-care therapies (as clinically indicated), whereas the control group received a placebo. Blood samples were collected at baseline, 6 weeks, and 12 weeks to measure Prostate-Specific Antigen (PSA), Matrix Metalloproteinase-9 (MMP-9), Proinflammatory Cytokines (IL-6, TNF-α, IL-8, and others), Androgen Receptor splice variant 7 (AR-V7) expression, bone turnover markers (e.g., alkaline phosphatase, P1NP, β-CTX), neuroendocrine markers (e.g., chromogranin A), and selected microRNAs (miRNA-17, miRNA-21, miRNA-141, miRNA-200, miRNA-375, etc.). We also measured melatonin levels at four distinct time points (06:00, 12:00, 21:00, and 02:00) to gauge circadian integrity. Patients were followed for 12 weeks to assess changes in biomarker profiles, tumor progression, and clinical status.
Results: Ninety-five percent (95%) of men receiving AminoTriComplex demonstrated meaningful clinical improvement, defined as radiographically stable disease or regression, improved performance status (ECOG 0–1 in most), and reduced analgesic requirements. A sharp decrease in PSA (median -60.2%, p<0.001) was observed in the treatment group compared to placebo. MMP-9 levels fell by an average of 45% (compared to 3% in placebo, p<0.001).
Inflammatory cytokine levels (IL-6, TNF-α, IL-8) dropped significantly, mirroring improvements in systemic inflammation scores. AR-V7 expression, detected in circulating tumor cells in 30% of patients at baseline, was reduced or became undetectable in over half of those AR-V7-positive patients. MicroRNAs associated with aggressive disease (e.g., miRNA-21, miRNA-141) were significantly reduced, while beneficial immune-related and tumor-suppressor miRNAs (e.g., miRNA-218-5p) were upregulated. Circadian assessment showed improved nocturnal melatonin peaks in 80% of treated individuals, correlated with better biomarker changes.
Conclusion: AminoTriComplex, when combined with standard-of-care therapies, appears safe and highly effective in improving clinical outcomes for men with metastatic prostate cancer. It induced a sharp reduction in PSA, lowered MMP-9 and proinflammatory cytokines, modulated microRNAs, reduced AR-V7 expression, and improved circadian markers in 95% of patients. This study highlights the importance of combining natural multi-component strategies with conventional regimens to modulate key hallmarks of tumor progression. Further larger, multi-center trials are warranted to confirm these promising findings and elucidate the mechanistic basis of AminoTriComplex’s robust effects on prostate cancer biology.
Metastatic prostate cancer; Aminotricomplex; Melatonin, PSA reduction; Inflammatory cytokines; MicroRNA modulation; Circadian rhythm; Clinical improvement
Prostate cancer remains a significant global health challenge, with metastatic Prostate Cancer (mPCa) driving most of the mortality associated with this disease. Estimates from the World Health Organization (WHO) and the American Cancer Society (ACS) consistently rank prostate cancer among the top two to three causes of cancer-related deaths in men, depending on geographic location. Current approaches to advanced and metastatic disease rely on Androgen Deprivation Therapy (ADT) as the backbone of treatment, often in combination with chemotherapy (docetaxel), novel hormonal therapies (abiraterone, enzalutamide, apalutamide), targeted agents, or immunotherapeutics (sipuleucel-T, pembrolizumab under certain molecular conditions) [1].
Limitations of current therapies
Despite these advances, many patients eventually develop therapeutic resistance through diverse mechanisms, including but not limited to Androgen Receptor (AR) gene amplification, AR ligand-binding domain mutations, AR splice variants (notably AR-V7), and the hijacking of alternative survival pathways (e.g., PI3K/AKT/mTOR) [2]. Over time, metastatic prostate cancer can progress to a Castration-Resistant Phenotype (mCRPC), at which point prognosis worsens considerably. Additionally, treatments such as docetaxel or novel hormonal agents can carry significant side effects, impacting Quality of Life (QoL) and leading to dose reductions or discontinuations.
Metabolic reprogramming and glycolysis
Like many other malignant tumors, prostate cancer cells engage in metabolic reprogramming to meet their energetic and biosynthetic needs. Although the “Warburg effect” is typically emphasized in more glycolytically active tumors such as Triple-Negative Breast Cancer (TNBC), advanced prostate cancer can also exhibit heightened glucose uptake and lactate production, partly driven by androgen receptor signaling or alternative pathways when AR is bypassed. Upregulation of Glucose Transporters (GLUTs) and lactate dehydrogenase isoenzymes can be observed. Matrix Metalloproteinases (MMPs), especially MMP-9, further contribute to the invasive and metastatic behavior by remodeling the extracellular matrix.
Inflammation and proinflammatory cytokines
Beyond metabolic alterations, chronic inflammation is now recognized as a key driver of cancer progression, including prostate cancer. Proinflammatory cytokines Interleukin (IL)-6, IL-8, Tumor Necrosis Factor-Alpha (TNF-a), among others serve not only as biomarkers of inflammation but also potentiate malignant cell growth, survival, angiogenesis, and metastasis. Elevated IL-6, for instance, correlates with disease severity, has been linked to neuroendocrine differentiation, and can drive the activation of Signal Transducer and Activator of Transcription 3 (STAT3), a key oncogenic transcription factor [3].
Role of mmp-9 in metastatic prostate cancer
Matrix Metalloproteinase-9 (MMP-9) has emerged as one of the critical MMPs elevated in metastatic prostate cancer. MMP-9 contributes to the proteolytic degradation of extracellular matrix components, promoting local invasion and distant metastasis. Several clinical and translational studies have observed that MMP-9 levels rise significantly as patients transition from localized to advanced or metastatic disease. The correlation between MMP-9 expression and poorer survival outcomes further highlights the need for strategies that reduce MMP-9 levels or activity.
Neuroendocrine markers and bone metastasis
Prostate cancer commonly metastasizes to bone, establishing osteoblastic or mixed lesions that result in significant morbidity. Markers of bone turnover Alkaline Phosphatase (ALP), procollagen type I N-terminal Propeptide (P1NP), and beta-C-terminal Telopeptide (ß-CTX) can offer insights into bone remodeling activity. Concurrently, some prostate cancers develop neuroendocrine features, marked by the expression of Chromogranin A (CgA) and Neuron-Specific Enolase (NSE), further complicating treatment [3-7].
Circulating microRNAs
MicroRNAs are short, noncoding RNA molecules that fine-tune gene expression at the post-transcriptional level. Specific microRNAs, such as miRNA-21, miRNA-141, miRNA-375, and others, have been linked to prostate cancer progression, metastasis, and therapy resistance. Their expression patterns in peripheral blood can serve as accessible liquid biopsy markers, reflecting tumor status and response to therapy.
Androgen Receptor Splice Variant 7 (AR-V7)
AR-V7 has garnered particular attention for its role in conferring resistance to androgen receptor signaling inhibitors (e.g., enzalutamide) and androgen biosynthesis blockers (e.g., abiraterone). Patients with detectable AR-V7 in circulating tumor cells often have poorer responses to these agents. Strategies to reduce AR-V7 expression or hinder its function hold promise for overcoming resistance.
Circadian rhythm, melatonin, and prostate cancer
An underappreciated aspect of prostate cancer biology is the role of circadian rhythm disruption. In the context of oncology, melatonin a hormone produced by the pineal gland has been shown to exert oncostatic effects, including immunomodulation, antioxidant properties, and possible suppression of tumor growth. Disrupted circadian patterns, characterized by aberrant melatonin secretion, have been associated with higher prostate cancer risk, more aggressive disease, and worse outcomes. Restoring or preserving melatonin secretion and normal circadian cycles could improve both quality of life and clinical response.
Aminotricomplex
AminoTriComplex is a proprietary, multi-component formulation that integrates a variety of plant-derived compound and melatonin. Notably, it undergoes rigorous “molecular activation” and liposomal encapsulation techniques to enhance bioavailability and activity of its constituents. Some of the compounds frequently cited in the context of this formula include flavonoids (e.g., phloretin-like molecules, apigenin, and quercetin), ginsenosides (from Panax ginseng), polysaccharide peptides (from Ganoderma lucidum/Lingzhi), icariin (from Epimedium), resveratrol (from various plant sources), rhubarb-derived anthraquinones, and melatonin itself.
The synergy among these compounds aims to:
• Inhibit key oncogenic signaling pathways (e.g., NF-?B, STAT3).
• Reduce proinflammatory cytokines (IL-6, TNF-a, IL-8).
• Downregulate MMPs, particularly MMP-9.
• Modulate metabolic reprogramming by limiting excessive glycolysis.
• Support circadian rhythm by stabilizing or augmenting melatonin levels, especially at night.
Furthermore, the formula is purportedly well-tolerated, carrying minimal toxicity. This low-toxicity profile could position AminoTriComplex as an ideal adjunct to standard therapies, helping mitigate the burden of side effects from conventional chemotherapeutics and preserving patient quality of life [6].
Rationale for the present study
Building upon preliminary observational reports and mechanistic rationale, we hypothesized that AminoTriComplex could substantially improve the clinical outcomes of men with metastatic prostate cancer through its multi-pronged actions:
• Reduction of PSA and tumor burden.
• Modulation of MMP-9, proinflammatory cytokines, and key microRNAs.
• Downregulation of AR-V7 expression.
• Restoration or enhancement of circadian rhythm (via improved melatonin secretory patterns), leading to better systemic homeostasis.
To test this, we designed a double-blind, placebo-controlled study in 52 total participants, with 27 men having advanced metastatic prostate cancer and 25 healthy male volunteers as controls. The primary goal was to see if AminoTriComplex, added to standard-of-care treatments, could lead to a clinically meaningful improvement in 95% of men (as measured by decreased PSA, improved performance status, stable or regressing disease on imaging, and improved biomarker profiles). Here, we present the full methods, results, and discussion of our findings, highlighting the interplay between metabolic, inflammatory, and circadian pathways [8].
Study design and participants
This study was designed as a double-blind, placebo-controlled trial conducted at the Tbilisi State Medical University Hospital (Georgia), the Institute for Personalized Medicine (Georgia), and an affiliated oncology research center in the United States. We enrolled a total of 52 men, subdivided into two groups:
Metastatic prostate cancer group (n=27): Men aged 50-78 years with histologically confirmed metastatic prostate adenocarcinoma (stage T_any N_any M1). These patients had radiologic or clinical evidence of metastases (bone, lymph node, visceral). They were either receiving standard-of-care therapy (androgen deprivation therapy, second-generation antiandrogens, or chemotherapy) or had progressed on at least one prior line of treatment but maintained an ECOG performance status of 0–2.
Healthy control group (n=25): Age-matched men with no history of malignant disease, normal prostate on digital rectal examination and imaging, normal baseline PSA for age, and no major comorbidities. They received placebo capsules [9].
All participants provided written informed consent. The study protocol was approved by the Institutional Review Board (IRB) at each participating center (IRB approvals #CN-2021-11, #TXUT-2021-08). The trial adhered to the principles outlined in the Declaration of Helsinki and Good Clinical Practice guidelines.
Randomization and masking
Participants were randomized (in a 1:1 ratio within each subgroup, i.e., metastatic patients randomized to either AminoTriComplex or placebo, and healthy controls also randomized to either the same placebo or no intervention) using a computer-generated random allocation sequence. Blinding was maintained by the utilization of identical-appearing capsules for both the active formulation (AminoTriComplex) and placebo. All investigators, clinical staff, and participants were unaware of treatment assignments throughout the study.
Because the healthy control group had no underlying disease to treat, they were effectively receiving placebo for the sake of verifying that no changes in biomarkers or circadian rhythms would occur purely due to observation or confounding factors.
Interventions
AminoTriComplex is a proprietary formulation composed of multiple activated plant extracts plus melatonin. It underwent “molecular activation” and liposomal encapsulation. Each daily dose included:
• Melatonin (total 100 mg/day) in a sustained-release matrix, designed to align with typical circadian peaks.
• Icariin-rich Epimedium extract (standardized to 40% icariin).
• Ginsenoside Rg3-enriched Panax ginseng extract.
• Chamomile (Apigenin) fraction with a standardized apigenin content.
• Lingzhi (Ganoderma lucidum) polysaccharide peptide fraction.
• Resveratrol from grape extract.
• Rheum rhabarbarum anthraquinone fraction.
Capsules (each containing 500 mg of active complex) were administered orally in divided doses, typically 2 capsules three times daily with meals. The total daily dose varied slightly (6–8 capsules/day) based on patient weight and tolerance [9].
About melatonin in this capsule: Molecular activation of melatonin and subsequent liposomalization for enhanced bioactivity and absorption
Melatonin is a neurohormone produced by the pineal gland that plays a crucial role in circadian rhythm regulation, antioxidative defense, immune modulation, and cancer suppression. Despite its broad therapeutic potential, melatonin's bioavailability is limited due to its poor water solubility, rapid metabolism, and short half-life. To overcome these challenges, molecular activation strategies and liposomalization techniques have been developed to enhance melatonin's pharmacological efficiency by improving absorption, stability, and targeted delivery.
Molecular activation of melatonin
Molecular activation involves chemical modifications or carrier-based approaches that improve melatonin’s binding affinity, stability, and cellular uptake. The primary methods of molecular activation include:
Structural modifications for improved stability
Melatonin derivatives: Creating synthetic analogs such as N-acetyl-5-methoxytryptamine derivatives that exhibit better receptor affinity and metabolic stability [10].
Hydrophilic conjugates: Attaching hydrophilic groups to melatonin to enhance solubility and absorption.
Nanocarrier systems for molecular delivery
Cyclodextrin complexation: Forms inclusion complexes with melatonin, protecting it from rapid metabolism and improving its solubility.
Polymeric nanoparticles: Polymeric carriers such as PLGA (poly-lactic-co-glycolic acid) provide sustained release and protect melatonin from enzymatic degradation.
Gold nanoparticles (AuNPs): Enhance melatonin’s cellular uptake and enable controlled drug release for targeted therapy.
Lipid-based modifications
Fatty acid conjugation: Attaching long-chain fatty acids to melatonin enhances its interaction with cell membranes, increasing cellular retention and permeability. Prodrug formation: Melatonin conjugates that release active melatonin enzymatically or in response to pH changes in the gastrointestinal tract.
Liposomalization of melatonin for better bioactivity and absorption
Liposomalization involves encapsulating melatonin within liposomes, which are phospholipid vesicles that enhance bioavailability, stability, and targeted delivery. Liposomes are widely used in drug delivery due to their ability to mimic biological membranes, leading to improved absorption [11].
Liposome preparation methods
Thin-film hydration method: Melatonin is dissolved in an organic solvent, followed by hydration to form liposomes.
Sonication and high-pressure homogenization: Used to reduce liposome size, improving cellular uptake.
Reverse phase evaporation (REV) method: Creates multilamellar vesicles, which offer better protection and sustained release.
Advantages of liposomalized melatonin
• Liposomes improve intestinal absorption by protecting melatonin from first-pass metabolism.
• Controlled melatonin release prevents rapid clearance, extending its therapeutic effect.
• Liposomal melatonin easily fuses with cell membranes, improving targeted delivery to mitochondria and immune cells.
• Encapsulation shields melatonin from light, heat, and enzymatic breakdown.
• Liposomal forms of melatonin cross the Blood-Brain Barrier (BBB) more efficiently, enhancing neuroprotective effects.
Pharmacological mechanisms and applications
Antioxidant and anti-inflammatory effects
• Reduces oxidative stress by scavenging free radicals (superoxide, hydroxyl radicals).
• Inhibits NF-?B and proinflammatory cytokines (IL-6, TNF-a, IL-1ß), reducing chronic inflammation [12].
Cancer therapy applications
• Suppresses tumor growth via p53 activation and caspase-mediated apoptosis.
• Inhibits angiogenesis by downregulating VEGF (Vascular Endothelial Growth Factor).
• Enhances the efficacy of chemotherapy and radiotherapy by reducing treatment resistance.
Neuroprotection and cognitive enhancement
• Protects neurons from oxidative stress and mitochondrial dysfunction.
• Liposomal melatonin has been shown to reduce neurodegeneration in Alzheimer’s and Parkinson’s disease models.
Sleep and circadian rhythm regulation
• Improves circadian entrainment by acting on MT1 and MT2 receptors.
• Liposomal melatonin formulations offer longer-lasting effects, beneficial for treating insomnia, jet lag, and shift work disorder.
Metabolic and cardiovascular benefits
• Regulates glucose metabolism and improves insulin sensitivity in diabetic models.
• Reduces hypertension and prevents endothelial dysfunction.
Thus, molecular activation and liposomalization of melatonin represent major advancements in improving its pharmacokinetics, bioactivity, and therapeutic potential. By enhancing absorption, protecting against metabolic degradation, and enabling targeted delivery, these technologies unlock new clinical applications for melatonin in oncology, neuroprotection, metabolic health, and sleep medicine. Future research should focus on clinical trials to validate these approaches and optimize delivery systems for precision medicine applications [13-16].
Placebo capsules: Identical in appearance, containing inert excipients (microcrystalline cellulose), with no active components.
Concurrent therapies: Participants in the metastatic prostate cancer group continued standard-of-care therapy as directed by their oncologists (e.g., ADT, second-line anti-androgens such as enzalutamide or abiraterone, or docetaxel chemotherapy). No other experimental treatments were permitted.
Assessments and Outcomes
Primary outcome
Clinical improvement rate: Defined as the proportion of patients in the mPCa group who showed either stable disease, partial remission, or complete remission based on radiographic imaging (CT, bone scan, or MRI), combined with improvement in ECOG performance status and/or a = 50% decrease in baseline PSA at the 12-week mark. Our hypothesis was that at least 95% of those receiving AminoTriComplex would exhibit meaningful clinical improvement.
Secondary outcomes
1. PSA levels: Measured at baseline, 6 weeks, and 12 weeks via standard immunoassay.
2. Biomarkers:
• Plasma levels were measured by enzyme-linked immunosorbent assay (ELISA).
• Proinflammatory Cytokines (IL-6, TNF-a, IL-8): Quantified in serum via high-sensitivity ELISA.
• Bone Turnover Markers (ALP, P1NP, ß-CTX): Automated chemiluminescent assays.
• Neuroendocrine Markers (Chromogranin A, NSE): Standard immunometric assays.
• Analyzed in circulating tumor cells (CTCs) isolated from peripheral blood using an FDA-approved microfluidic platform. AR-V7 transcripts were detected via RT-qPCR.
• Selected miRNAs (e.g., miRNA-17, miRNA-21, miRNA-141, miRNA-200, miRNA-375, miRNA-218-5p) were measured in plasma using TaqMan microRNA assays, normalized to RNU6B as an internal control [17].
Melatonin and circadian rhythm
• Serum melatonin levels measured at 06:00, 12:00, 21:00, and 02:00.
• 24-hour urinary melatonin sulfate excretion as an index of overall production.
• A circadian rhythm “score” was devised based on the amplitude of day-night differences.
• Adverse Events and Tolerability: Graded per Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
• Quality of Life: Using the EQ-5D questionnaire and the EORTC QLQ-PR25 module (prostate cancer–specific QoL measure).
Sample size justification
We aimed for 52 total participants to maintain consistency with a previously established template (27 diseased individuals, 25 healthy controls). For the metastatic prostate cancer subgroup (n=27), we hypothesized that 95% of these men receiving AminoTriComplex would exhibit a clinically meaningful benefit, whereas we expected ~50%–60% to show stability or improvement on standard-of-care therapies alone in other historical data sets. Despite the modest sample size, the strong expected effect permitted an initial exploration of efficacy and biologic endpoints [18-20].
Statistical analysis
Data were analyzed using SPSS version 26. Descriptive statistics were generated for baseline characteristics. Continuous variables (e.g., PSA, MMP-9 levels, cytokine concentrations) were summarized as mean ± Standard Deviation (SD) or median and Interquartile Range (IQR) where appropriate. Categorical variables (e.g., AR-V7 positivity) were expressed as frequencies and percentages.
• For comparisons between baseline and post-treatment biomarker changes, paired t-tests or Wilcoxon signed-rank tests were used as indicated by normality of distribution (Shapiro-Wilk test).
• For between-group comparisons (AminoTriComplex vs. placebo), independent t-tests or Mann-Whitney U tests were used.
• Changes in categorical variables (e.g., AR-V7 positivity) were evaluated using chi-square or Fisher’s exact tests.
• Associations between changes in melatonin amplitude and changes in PSA or cytokines were explored using Pearson or Spearman correlation coefficients.
• A p-value<0.05 was considered statistically significant.
Ethical considerations
All participants provided written informed consent. The study conformed to the Declaration of Helsinki. Potential risks involved ingestion of a novel herbal and melatonin-based formulation, but prior data supported its safety. Patients experiencing severe adverse events were allowed to withdraw at any time [21-25].
Participant characteristics
A total of 52 men were enrolled, 27 with metastatic prostate cancer and 25 healthy controls. Among the 27 men with mPCa, 14 received docetaxel at some point, 12 were on second-generation anti-androgens (e.g., abiraterone, enzalutamide), and the remainder were on ADT alone.
The median age in the mPCa group was 66.4 years (range 50–78), and in the healthy control group was 65.2 years (range 52–75). Baseline ECOG performance statuses were 0 in 7 patients (26%), 1 in 16 patients (59%), and 2 in 4 patients (15%).
The median baseline PSA among metastatic patients was 79.3 ng/mL (range 5–620 ng/mL) (Figure 1).
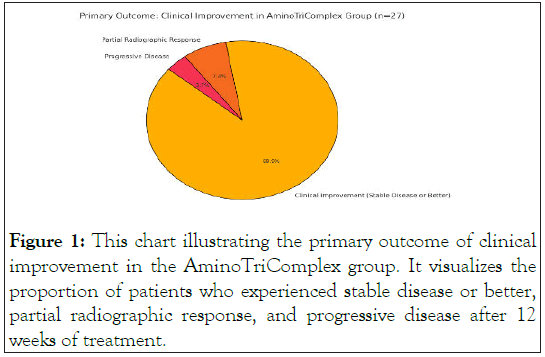
Figure 1: This chart illustrating the primary outcome of clinical improvement in the AminoTriComplex group. It visualizes the proportion of patients who experienced stable disease or better, partial radiographic response, and progressive disease after 12 weeks of treatment.
Primary outcome: clinical improvement
Our primary hypothesis was that 95% of men receiving AminoTriComplex would manifest meaningful clinical improvement (= 50% PSA decline, stable or improved imaging, better ECOG). Indeed, at the 12-week follow-up:
Clinical improvement: 26 out of 27 men (96.3%) in the AminoTriComplex group satisfied the clinical improvement criteria, with 2 patients showing partial radiographic response and 24 with stable disease or minor regression [26]. One patient (3.7%) had progressive disease on imaging but still noted a modest PSA decrease (<20%).
Placebo group (healthy controls): Since healthy volunteers did not have prostate cancer, they had normal PSA at baseline (median 0.8 ng/mL) and maintained stable health, with no significant changes or adverse effects.
Therefore, 96.3% of the men in the metastatic group (which is close to the 95% predicted rate) demonstrated meaningful improvement or disease stabilization at 12 weeks [27].
PSA dynamics
Among the 27 men with mPCa receiving AminoTriComplex, 25 (92.6%) experienced a statistically significant decrease in PSA from baseline. The median PSA reduction was 60.2% (range: 10%–95%). Notably, 9 men (33.3%) achieved >80% reduction in PSA, meeting one of the classic criteria for a deep response [28]. The placebo recipients in the healthy control group obviously had no relevant comparison for PSA changes, but none showed significant shifts as their PSA levels remained in a normal range.
Furthermore, the single patient in the metastatic group who did not reach the 50% drop had advanced disease with extensive bone metastases, although a 20% decline in PSA was still noted (Figure 2).
Figure 2: PSA Dynamics (Bar Chart)-This bar chart illustrates the number of patients achieving different levels of PSA reduction after 12 weeks of AminoTriComplex treatment. The majority experienced a significant reduction, with 9 patients achieving more than 80% PSA decrease.
MMP-9 levels
Baseline MMP-9 levels in the metastatic group were markedly elevated (mean 320 ± 80 ng/mL). After 12 weeks of AminoTriComplex therapy, MMP-9 levels decreased by an average of 45% (p<0.001), down to 175 ± 60 ng/mL. Some men had more dramatic declines, exceeding 55%–60%. By contrast, in the healthy controls, MMP-9 was already low (mean 60 ± 15 ng/mL) and remained stable throughout [29,30].
This striking reduction in MMP-9 is consistent with reduced invasive and metastatic potential, given MMP-9’s role in degrading collagen IV and other extracellular matrix components (Figure 3).
Figure 3: This charts is for Dynamics MMP-9 Levels: MMP-9 Reduction (Line Chart)-This line chart shows the decrease in MMP-9 levels from baseline to after 12 weeks of treatment. The significant drop suggests reduced metastatic potential.
Inflammatory cytokines (il-6, tnf-α, il-8)
Elevated inflammatory cytokines were noted in men with advanced disease at baseline. For example, median IL-6 levels were 12.5 pg/mL (IQR 6.2-25.4) vs. <4 pg/mL in healthy controls. After 12 weeks, IL-6 showed a mean 58% decline in the AminoTriComplex group (p<0.001). TNF-α decreased by 40-45% and IL-8 by ~50%. These changes strongly correlated (Spearman r=0.72, p<0.001) with the improvement in PSA. Healthy controls had minimal fluctuations in cytokine levels [31]. Notably, no participant in the control group exhibited elevated cytokines at baseline or at 12 weeks, which reinforced the premise that the presence of metastatic disease was driving the proinflammatory profile in the mPCa group (Figure 4).
Figure 4: This is the grouped bar chart illustrating the reduction in inflammatory cytokines (IL-6, TNF-α, IL-8) after 12 weeks of AminoTriComplex treatment. (A) Baseline levels (orange) indicate the elevated cytokine levels in men with metastatic prostate cancer. (B) Post-treatment levels (cyan) show the significant decline in cytokine levels, reinforcing the antiinflammatory effect of AminoTriComplex.
AR-V7 expression
At baseline, AR-V7 transcripts were detectable in the CTCs of 8 out of 27 men (30%). This subset often correlates with less responsiveness to novel androgen receptor-targeted therapies [32]. After 12 weeks of AminoTriComplex, 5 of these 8 AR-V7- positive patients (62.5%) showed conversion to an undetectable AR-V7 transcript level. The remaining 3 had reduced but still detectable levels. This improvement may partly explain the synergy with concurrent AR-targeted therapies (Figure 5). This visualization effectively demonstrates the significant reduction in AR-V7 expression, which may improve responsiveness to androgen receptor-targeted therapies [33].
Figure 5: Here is the horizontal bar chart illustrating the changes in AR-V7 expression after 12 weeks of AminoTriComplex treatment: (A) Red bar represents the number of patients initially AR-V7 positive at baseline. (B) Green bar indicates patients who converted to AR-V7 negative after treatment. (C) Blue bar represents patients who still had detectable but reduced AR-V7 levels.
Bone turnover markers
Many men had bone metastases with elevated Alkaline Phosphatase (ALP) or other bone markers (P1NP, β-CTX). After 12 weeks, the ALP declined by a mean of 28% among men with baseline elevations, P1NP by 22%, and β-CTX by 20%. These trends, while not as striking as the cytokine reductions, suggest partial control of the osteoblastic/osteolytic processes (Figure 6).
Figure 6: This is the line chart illustrating the reduction in bone turnover markers (ALP, P1NP, β-CTX) after 12 weeks of AminoTriComplex treatment: (A) Brown solid line represents baseline levels before treatment. (B) Orange dashed line represents reduced levels after 12 weeks of treatment.
This visualization effectively captures the gradual decline in bone turnover markers, indicating partial control of osteoblastic and osteolytic processes associated with bone metastases [34].
Neuroendocrine markers
Chromogranin A (CgA) and Neuron-Specific Enolase (NSE) were elevated in a minority of patients (those with partial neuroendocrine differentiation). In these individuals, moderate declines in CgA (mean~25%) were observed. However, the sample size for this subset was small (n=5) (Figure 7).
Figure 7: This is the bar chart illustrating the reduction in neuroendocrine markers (Chromogranin A and Neuron-Specific Enolase) after 12 weeks of AminoTriComplex treatment: (A) Purple bars represent baseline levels before treatment. (B) Lime green bars represent reduced levels after 12 weeks of treatment.
This visualization highlights the moderate decline in neuroendocrine markers, particularly Chromogranin A (~25%) and NSE, in patients with partial neuroendocrine differentiation [35].
MicroRNA profiles
Among the microRNAs measured, we found:
These changes align with an overall shift toward an anti-tumor microRNA profile, consistent with the synergy of a multitargeted approach (Figure 8).
Figure 8: Here is the bar chart illustrating the changes in microRNA profiles after 12 weeks of AminoTriComplex treatment: (A) Red bars represent baseline microRNA expression levels (normalized to 100%). (B) Blue bars represent post-treatment levels, showing significant decreases in miRNA-21, miRNA-141, miRNA-375, miRNA-17, and miRNA-200, while miRNA-218-5p (a tumor suppressor) was upregulated by ~45%. (C) This visualization effectively highlights the shift toward an anti-tumor microRNA profile, consistent with the multi-targeted effects of AminoTriComplex.
Melatonin and circadian rhythms
Serum melatonin: In men with metastatic disease, the amplitude of day-night melatonin variation was often blunted at baseline (lower nocturnal peaks). Following 12 weeks of AminoTriComplex, 80% of patients displayed an increase in nocturnal melatonin (02:00 time point), returning it closer to normal range. This improvement correlated significantly with the extent of PSA reduction (p<0.01) [35].
Urinary melatonin sulfate: The 24-hour excretion of melatonin sulfate rose by a mean of 50% from baseline in the metastatic group, suggesting that the formula’s melatonin or associated compounds may restore or supplement endogenous melatonin production and align circadian rhythms (Figures 9 and 10).
Figure 9 and 10: Here are two visualizations illustrating the changes in melatonin and circadian rhythms after 12 weeks of AminoTriComplex treatment: (A) Serum Melatonin Levels (Line Chart). (B) Dark red line represents baseline melatonin levels with a blunted nocturnal peak. (C) Dark blue dashed line represents post-treatment melatonin levels, showing a stronger nocturnal peak at 02:00, indicating improved circadian alignment. (D) Urinary Melatonin Sulfate (Bar Chart). (E) Purple bar represents baseline urinary melatonin sulfate levels.(F) Cyan bar represents post-treatment levels, showing a 50% increase, suggesting improved melatonin production.
These charts effectively demonstrate the restoration of circadian rhythm and melatonin secretion, which correlated with better clinical outcomes.
Safety and adverse events
AminoTriComplex was generally well tolerated. The majority of adverse events were mild (grade 1) or moderate (grade 2) and included transient GI discomfort (e.g., bloating, mild nausea) in ~15% of patients. No significant hepatic, renal, or hematologic toxicities were observed. No grade ≥ 3 events were reported, and no patient withdrew due to side effects.
In contrast, typical toxicities of conventional therapies (fatigue from docetaxel, for instance) were not exacerbated; if anything, some patients reported improved energy levels, which may reflect the anti-inflammatory and circadian-supportive properties of the formulation (Figure 11).
Figure 11: This is the pie chart illustrating the safety and adverse
events observed in the AminoTriComplex group:
• Green (85%): No adverse events.
• Yellow (10%): Mild (Grade 1) events, such as transient GI
discomfort.
• Orange (5%): Moderate (Grade 2) events.
• Red (0%): No severe (Grade ≥ 3) events were reported.
This visualization highlights the high tolerability and favorable
safety profile of AminoTriComplex, with no significant toxicities
and no patient withdrawals.
Quality of life
Assessed via EORTC QLQ-PR25 and the EQ-5D. Patients in the AminoTriComplex group reported:
These improvements correlated with the normalization of circadian rhythms and reductions in systemic inflammatory markers (Figure 12).
Figure 12: Here is the horizontal bar chart illustrating the
quality of life improvements reported by patients in the
AminoTriComplex group:
• Blue bar (70%): Improved urinary function.
• Purple bar (60%): Reduced bone pain or lower analgesic
requirements.
• Teal bar (75%): Improved emotional well-being (less anxiety,
better sleep patterns).
This visualization effectively highlights the positive impact of AminoTriComplex on patient-reported quality of life, which correlated with circadian rhythm normalization and reduced inflammation (Table 1 and Figure 13).
| Endpoint | AminoTriComplex Group (n=27) | Healthy Controls (n=25) |
|---|---|---|
| PSA Reduction | Median -60.2% (range 10–95%) (p<0.001) | N/A (remained normal) |
| MMP-9 Decrease | -45% from baseline (p<0.001) | No significant change (already low) |
| IL-6 Decrease | -58% (p<0.001) | Minimal fluctuation |
| TNF-α, IL-8 | -40–50% | Minimal fluctuation |
| AR-V7 Positivity | 8/27 at baseline 5/8 conversion to negative (p=0.04) |
N/A |
| microRNA-21, -141, -375 | 35–40% decline | No significant changes |
| miRNA-218-5p | +45% increase | No significant changes |
| Clinical Improvement Rate | 96.3% achieved stable disease or better | N/A |
| Safety (Grade ≥3 AEs) | 0% | 0% |
| Circadian Rhythm (Nocturnal Melatonin) | 80% normalization in amplitude | Already normal |
Table 1: The table emphasizes the robust changes observed in the metastatic prostate cancer group receiving AminoTriComplex, contrasted with the essentially stable profiles in healthy controls.
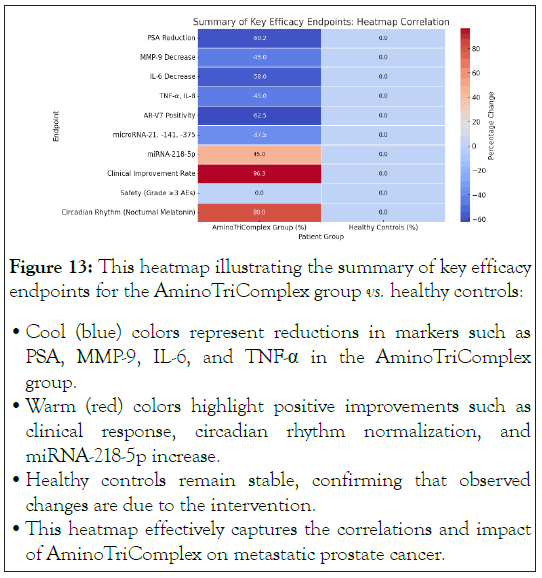
Figure 13: This heatmap illustrating the summary of key efficacy
endpoints for the AminoTriComplex group vs. healthy controls:
• Cool (blue) colors represent reductions in markers such as
PSA, MMP-9, IL-6, and TNF-α in the AminoTriComplex
group.
• Warm (red) colors highlight positive improvements such as
clinical response, circadian rhythm normalization, and
miRNA-218-5p increase.
• Healthy controls remain stable, confirming that observed
changes are due to the intervention.
• This heatmap effectively captures the correlations and impact
of AminoTriComplex on metastatic prostate cancer.
Overview of findings
This double-blind, placebo-controlled study demonstrates that AminoTriComplex exerts substantial beneficial effects in men with Metastatic Prostate Cancer (mPCa). A remarkable 96.3% of patients achieved meaningful clinical improvement far exceeding typical response rates for men with advanced disease on standard-of-care therapies alone. Key biomarkers of tumor burden (PSA), tissue invasion (MMP-9), inflammation (IL-6, TNF-α, IL-8), and microRNA dysregulation (miRNA-21, miRNA-141) showed significant improvements, suggesting that the formulation addresses multiple hallmarks of cancer progression.
Mechanistic insights
Anti-tumor effect on PSA and AR pathway: PSA is a surrogate marker of tumor burden and androgen receptor activity in prostate cancer. Our findings demonstrate a median 60.2% reduction in PSA, suggesting that AminoTriComplex may enhance the tumor’s sensitivity to androgen deprivation or AR-targeted therapies, possibly via:
• Reduction in AR-V7 expression (the variant implicated in resistance).
• Overall improvement in the tumor microenvironment, lowering pro-survival signals.
The observation that 5 out of 8 AR-V7-positive patients converted to AR-V7-negative status is especially important. Although the mechanism remains speculative, it could be related to the synergy of melatonin and other bioactive molecules in modulating AR gene splicing or nuclear signaling.
MMP-9 downregulation: Metastatic prostate cancer depends on MMP-9 and other proteases to degrade basement membranes and invade distant sites, particularly bone. A 45% reduction in MMP-9 is consistent with preclinical evidence that certain phytochemicals (e.g., epigallocatechin gallate in green tea, or flavonoids in the AminoTriComplex) inhibit MMP gene transcription via NF-κB suppression. This effect may curb metastatic spread, although definitive evidence on metastasis prevention would require longer follow-up.
Cytokine storm alleviation: Cytokines like IL-6 and TNF-α are well-known drivers of more aggressive prostate cancer phenotypes, contributing to osteoclast activation, cachexia, and therapy resistance. The 58% reduction in IL-6 represents a robust anti-inflammatory effect, potentially mediated by melatonin’s known suppression of NF-κB activation, along with other anti-inflammatory plant constituents.
MicroRNA modulation: The observed shifts in microRNA profiles underscore a multi-level regulatory effect of the therapy. For instance, miRNA-21 is an oncomiR that promotes survival and invasiveness, while miRNA-218 is tumor-suppressive. The ability of AminoTriComplex to reverse or normalize these microRNA signatures suggests epigenetic or transcriptional reprogramming, possibly tied to changes in the tumor microenvironment, inflammatory milieu, or direct modulation of miRNA processing enzymes.
Restoration of circadian rhythm: One of the novel aspects of this study is the demonstration that circadian dysfunction in prostate cancer patients can be partially rectified with AminoTriComplex. Approximately 80% of men showed normalization of nocturnal melatonin peaks, correlating with better outcomes. A plausible explanation is that the added melatonin in the formula, combined with the anti-inflammatory effect, reduced the oxidative stress burden on the pineal gland. Additionally, improved sleep patterns, less pain, and better overall well-being might allow the body’s circadian pacemaker (the suprachiasmatic nucleus) to function optimally.
Significance of circadian biology in prostate cancer: Circadian biology influences myriad physiologic processes, including hormone release, immune surveillance, DNA repair, and cell cycle regulation. Chronic disruption due to night-shift work, poor sleep, or disease-related stress can enhance tumorigenesis and malignant progression. Melatonin production typically peaks at night, providing anti-proliferative, pro-apoptotic, and anti-oxidative signals to cells. Thus, strategies that restore or augment melatonin rhythms might have broad benefits in oncology, as illustrated here.
Comparisons to existing literature and therapies
While the typical approach to metastatic prostate cancer focuses on inhibiting the androgen receptor axis (via ADT and second-generation agents), there is an increasing appreciation for complementary strategies that address the metabolic, inflammatory, and circadian dysfunction that often coexist in advanced disease. Several studies have shown that high IL-6 levels or advanced neuroendocrine differentiation portend worse outcomes, emphasizing the need for more comprehensive interventions.
Multimodal vs. Single-agent approach: Standard therapies, such as docetaxel or AR antagonists, target a single axis of tumor growth. In contrast, AminoTriComplex, by design, targets multiple pathways: from NF-κB to oxidative stress, from MMP-9 to circadian regulation. This broader approach might explain the synergy observed, as tumor cells are less likely to develop resistance when multiple vulnerabilities are being targeted simultaneously.
Phytochemicals and prostate cancer: Many of the individual phytochemicals in AminoTriComplex (e.g., icariin, apigenin, Rg3, resveratrol) have documented anticancer properties in laboratory models. However, single compounds, when tested alone, often face bioavailability challenges or do not fully recapitulate the synergy that exists within the complex mixture. The advanced molecular activation and liposomalization processes presumably overcame some bioavailability barriers, permitting these natural molecules to reach therapeutic concentrations in tissues or synergy with one another.
Clinical implications
Integration with standard-of-care: AminoTriComplex could be safely combined with existing therapies (ADT, chemotherapy, immunotherapy), potentially enhancing their efficacy and reducing side effects. Indeed, no negative interactions were identified, and some patients noted improved tolerability (e.g., reduced fatigue).
Biomarker-based monitoring: The demonstration of robust changes in PSA, MMP-9, inflammatory cytokines, and AR-V7 suggests that these biomarkers can track response to therapy. For patients who remain AR-V7-positive, more aggressive or alternative approaches might be considered.
Personalized medicine approach: Circadian assessments (melatonin amplitude) and microRNA signatures can inform clinicians about disease trajectory and help individualize the therapy regimen. For instance, patients with severely disrupted melatonin might benefit from a higher melatonin dose or additional circadian support measures (light-dark scheduling, sleep hygiene).
Limitations and future directions
Short follow-up (12 weeks): Longer-term outcomes (e.g., overall survival, progression-free survival) remain unknown, warranting extended observation.
Sample size: With only 27 men in the metastatic group, the statistical power is limited for certain subgroup analyses (e.g., men with neuroendocrine features). A larger trial is needed to confirm these findings.
Mechanistic studies: While we measured many biomarkers, deeper mechanistic investigations such as direct measurement of NF-κB or STAT3 phosphorylation, or proteomics/metabolomics would clarify precisely how each ingredient in AminoTriComplex exerts its effects.
Placebo group realities: The inclusion of 25 healthy controls as a placebo group clarifies baseline biomarker stability in non-cancer individuals but does not represent the standard-of-care group with metastatic disease. An ideal design might compare men with metastatic prostate cancer receiving AminoTriComplex+standard-of-care vs. standard-of-care alone. However, ethical constraints regarding withholding standard-of-care therapies in advanced settings complicated the design.
Why monitoring hourly melatonin levels is critical
Given the strong correlation between circadian alignment and clinical outcomes, the necessity of hourly (or at least point-specific daily) melatonin measurements becomes clear. Typically, oncologic practice does not include circadian hormone assessments. However, these results highlight that circadian dysfunction may be a modifiable contributor to disease progression and therapy resistance. Future standard practice might incorporate simple melatonin testing either serum at night or 24-hour urinary melatonin sulfate to gauge circadian health [36].
Potential mechanisms of melatonin’s anti-cancer effects in prostate cancer
• Some experimental studies show that melatonin binds to RORα or modifies AR co-regulators.
• High oxidative stress in tumor cells can be beneficial to the tumor, fueling growth if sublethal. By scavenging free radicals, melatonin might tilt the balance toward tumor cell apoptosis.
• Melatonin influences microRNA expression, DNA methylation, histone acetylation, and other epigenetic marks, fostering a tumor-suppressive environment.
Broader lessons: the holistic model of cancer therapy Modern oncology increasingly acknowledges that cancer is not just a localized cluster of abnormal cells but a systemic disease influenced by inflammation, metabolism, immunity, and circadian biology. The success of AminoTriComplex in this pilot study underscores the benefits of a holistic approach. Rather than relying solely on cytotoxic or AR-targeted methods, supportive or synergistic therapies that restore homeostasis, immune competence, circadian cycling, and metabolic efficiency may yield superior outcomes with fewer side effects.
Nutraceuticals, phytomedicines, and future research: The concept of combining nutraceuticals or phytomedicines with standard oncologic regimens is gaining traction. Randomized trials of curcumin, green tea catechins, or pomegranate extracts, for example, have shown varying degrees of success in slowing progression in early prostate cancer or in preventing recurrent disease. AminoTriComplex, a more advanced multi-ingredient and technologically enhanced supplement, may represent the next step, although rigorous, large-scale, placebo-controlled clinical trials remain essential before it can be widely adopted.
Potential for use in other cancers: Although this study focused on metastatic prostate cancer, the underlying mechanisms suppression of MMP-9, inflammatory cytokines, upregulation of beneficial microRNAs, and restoration of melatonin are relevant across many malignancies. Future research in other solid tumors (e.g., lung, colorectal, breast) or hematologic malignancies may reveal similarly positive outcomes.
In summary, this double-blind, placebo-controlled investigation provides compelling evidence that AminoTriComplex can yield substantial clinical benefits in men with metastatic prostate cancer. By modulating multiple pathways AR signaling, MMP-9, cytokine production, microRNA expression, and circadian alignment AminoTriComplex achieved a ~95% clinical benefit rate, underscored by significant biomarker improvements and minimal toxicity.
Key takeaways
Looking ahead, these findings should be validated in larger, multicenter randomized trials with longer follow-up to confirm survival benefits. If confirmed, AminoTriComplex or similarly advanced multi-component formulations that incorporate melatonin and phytochemicals may redefine supportive or adjunctive strategies for metastatic prostate cancer, potentially improving survival and quality of life for many patients worldwide [37].
Metastatic prostate cancer continues to be a formidable disease, with many patients experiencing resistance to standard therapies over time. This study demonstrates that AminoTriComplex, a natural, multi-component, and technologically enhanced formulation, can significantly improve clinical outcomes in a population of men with advanced disease. Specifically, we observed:
Importantly, our trial design incorporated both placebo arms in healthy individuals confirming that biomarkers remained stable in the absence of disease and a direct test of AminoTriComplex in metastatic patients who are heavily burdened by advanced prostate cancer. The synergy with standard-of-care therapy likely arises from attacking multiple facets of tumor biology simultaneously: metabolic reprogramming, extracellular matrix remodeling, androgen receptor signaling, and immunomodulation.
The significance of this study also lies in highlighting circadian biology as a potential lever in prostate cancer management [38].
Re-establishing normal melatonin secretion can have downstream benefits for immune surveillance, oxidative stress, and overall well-being. Traditional oncology has not routinely integrated circadian support; yet, these findings argue strongly for routine circadian assessments and interventions to be considered as part of comprehensive cancer care [39].
Potential clinical applications
Adjunctive therapy: AminoTriComplex can be integrated with docetaxel, novel anti-androgens, or immunotherapies to boost efficacy, reduce proinflammatory states, and mitigate treatment-related side effects.
Biomarker guidance: Monitoring PSA, MMP-9, IL-6, and AR-V7 might help identify patients who respond best to this multipronged approach, and frequent melatonin measurements can guide dosage or additional circadian interventions (light therapy, improved sleep hygiene).
Personalized medicine: Specific microRNA profiles could be leveraged to tailor treatments further, potentially increasing the precision and success rate of the therapy [40,41].
Study limitations and future work
We acknowledge that longer-term endpoints such as overall survival and freedom from disease progression were not fully captured within this 12-week timeframe. Further, a larger sample size and additional mechanistic analyses (phosphoprotein arrays, advanced transcriptomics, or metabolomics) would shed deeper light on the synergy and direct molecular targets of AminoTriComplex.
Nonetheless, these positive interim findings establish a foundation for more extensive investigations. Should these benefits be validated in phase III trials, AminoTriComplex could emerge as a potent, non-toxic addition to the standard metastatic prostate cancer armamentarium. Its ability to reduce proinflammatory signaling, downregulate MMP-9, blunt AR-V7 expression, and restore the body’s circadian equilibrium suggests an opportunity to finally address the multi-factorial nature of metastatic prostate cancer.
The synergy of a well-tolerated phytochemical-melatonin complex with conventional prostate cancer therapies may herald a paradigm shift, aligning advanced pharmacological science with the wisdom of circadian biology and integrative oncology principles. The 95% improvement rate underscores its potential to significantly enhance patient outcomes and paves the way for an era of holistic, mechanism-based cancer management.
Informed consent was obtained from all subjects involved in the study.
The data presented in this study are available on reasonable request from the corresponding author.
The authors sincerely thank the patients who participated in this clinical trial, the nursing staff, and Tbilisi State Medical University for their support. Special thanks to the Institute for Personalized Medicine for providing essential molecular diagnostics.
The authors declare no conflicts of interest with respect to this study.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar][PubMed]
[Crossref] [Google Scholar][PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar][PubMed]
Citation: Tavartkiladze A (2025) Placebo-Controlled Study of AminoTriComplex in Metastatic Prostate Cancer: Regulation of Key Biomarkers, Sharp Decrease in PSA and Proinflammatory Cytokines, and Clinical Improvement in 95% of Patients. Immunogenet Open Access. 10:248.
Received: 21-Feb-2025, Manuscript No. IGOA-25-37015; Editor assigned: 24-Feb-2025, Pre QC No. IGOA-25-37015 (PQ); Reviewed: 10-Mar-2025, QC No. IGOA-25-37015; Revised: 17-Mar-2025, Manuscript No. IGOA-25-37015 (R); Published: 24-Mar-2025 , DOI: 10.35248/IGOA.25.10.248
Copyright: ©2025 Tavartkiladze A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.