Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Short Communication - (2024)Volume 13, Issue 1
Castration-Resistant Prostate Cancer (CRPC) often has genetic alterations in the Androgen Receptor (AR) signaling pathway. Typically, CRPC drugs work by either curbing dihydrotestosterone biosynthesis or impeding AR signaling. In this mini-review, we discuss Drug-Tolerant Persister (DTP), a phenotypic state that is reversible and without genetic mutation on the AR. Persister cells may proffer a new perspective on the development of CRPC.
Androgen Deprivation Therapy (ADT); Androgen receptor inhibitor; Castration-Resistant Prostate Cancer (CRPC); Drug Tolerant Persister (DTP)
The clinical challenge of prostate cancer
Prostate Cancer (PCa) incidence and mortality are estimated to increase from 1.41 and 0.375 million in 2020 to 2.43 and 0.74 million in 2040 globally. Since Dr. Charles Huggins’ discovery in 1941, Androgen Deprivation Therapy (ADT) has been the mainstay to control the spread of advanced prostate cancer 1. Despite the initial success, nearly all patients progress to Castration-Resistant Prostate Cancer (CRPC) and subsequently succumb to the disease within 1-3 years [1-8].
Androgen Deprivation Therapy (ADT)
ADT is achieved typically by either androgen level reduction through castration (orchiectomy, gonadotropin-releasing hormone analog, abiraterone, ODM-2085), Androgen Receptor (AR) blockade using AR inhibitor (flutamide, bicalutamide, nilutamide 8, enzalutamide, apalutamide, darolutamide, rezvilutamide) or a combination of both Significant efforts have been made over the last 80 years, yet the castration resistance issue remains unsettled [9-12], (Figure 1).
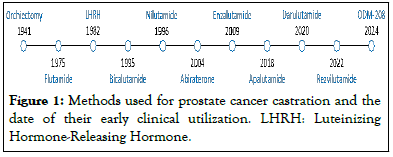
Figure 1: Methods used for prostate cancer castration and the date of their early clinical utilization. LHRH: Luteinizing Hormone-Releasing Hormone.
Mechanisms of castration resistance
Initially, the failure of ADT was thought to be due to a “hormone refractory” state. Evidence indicates, however, that AR pathway activation plays a pivotal role in most cases of “hormone refractory” cancers, and therefore the term Castration-Resistant Prostate Cancer (CRPC) was proposed [13]. CRPC can surmount AR signaling inhibition by various genetic and epigenetic mechanisms: e.g., AR overexpression and gene amplification, AR ligand-binding domain mutations, gene rearrangements, constitutively active AR variants, and alterations in pathways of androgen biosynthesis [14]. Although secondgeneration drugs, such as enzalutamide binds to AR with greater affinity than bicalutamide and abiraterone blocks the adrenal source of androgen synthesis, can overcome the primary resistance, castration resistance will recur after their extended use [15,16]. These cancer cells may also be cross-resistant to apalutamide and darolutamide [17]. In addition, utilization of second-generation AR inhibitors may force cancer cells to survive via AR-independent pathways, for instance, neuroendocrine prostate cancer [18].
DTP cell and CRPC
The above-mentioned drug resistance often involves genetic alterations related to the AR signaling pathway. However, castration resistance may also be acquired through a reversible drug tolerance, a phenotypic state that no longer depends on the drug-targeted pathway. We have recently observed that the Drug-Tolerant Persister (DTP) state is responsible for AR inhibitor resistance in vitro and CRPC development in vivo [19]. The notion of DTP originates from microbiology, where a subpopulation of bacteria can survive in antibiotics without initially being genetically resistant [20]. In 2010, drug-tolerant persister cancer cells were reported, which had enhanced drug resistance, did not carry genetic mutations, and were phenotypically reversible after drug withdrawal [21]. This original study revealed a small fraction of viable quiescent cells that survived the treatment with a drug concentration of 100- fold greater than the IC50 for 9 days (DTP) and that resumed proliferation in continuous drug exposure for 33 days (Drug- Tolerant Expanded Persister or DTEP) .
DTP cells have been observed in many cancer types including cancer of the lung, breast, ovary, colorectum, stomach, head and neck, pancreas, and prostate as well as melanoma, and leukemia, and considered as a main form of cancer cell resistance to chemotherapeutic drugs [22-28]. It is noteworthy that Neuro Endocrine (NE)-like cell biomarkers (CgA, CgB, NSE, and PTHrP) are also increased in DTP cells [19]. Therefore, the DTP model may well recapitulate the clinical course of CRPC where both AR-positive carcinoma and AR-negative neuroendocrine cancer cells develop [29-31].
Lipid peroxidation and drug resistance
Several mechanisms, namely epigenomic modification, transcriptional regulations, tumor microenvironment, and metabolic remodeling, have been reported to regulate cancer persister state [32]. Metabolic remodeling, specifically lipid peroxidation, seems to play a key role in the case of AR inhibitor-resistant persister cells. AR inhibitor (enzalutamide, EPI-001, darolutamide) treatment gives rise to DTP and the thioredoxin/peroxiredoxin pathway is up-regulated [19]. Peroxiredoxin 5 (PRDX5) promotes AR inhibitor resistance and CRPC development. Inhibition of PRDX5 quells DTP cell proliferation in culture, delays CRPC development in animal models, and stabilizes Prostate-Specific Antigen (PSA) progression and metastatic lesions in patients. PRDX5 is an antioxidant enzyme and its peroxidic Cys48 thiol (Cys48-SH) is oxidized to sulfenic acid (Cys48-SOH) while reducing hydrogen peroxide and alkyl hydroperoxides. The Cys152 on PRDX5 then reacts with Cys48-SOH to form an intramolecular disulfide. Thioredoxins reduce the disulfide bond, regenerate a sulfhydryl group on the Cys48, and thereby reactivate PRDX5 in a NADPH-dependent manner [33], (Figure 2).
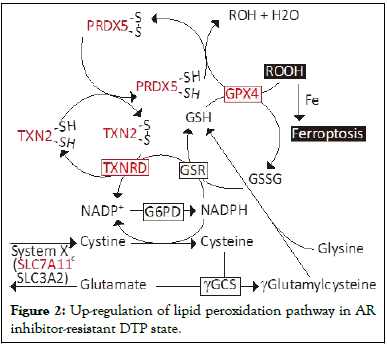
Figure 2: Up-regulation of lipid peroxidation pathway in AR inhibitor-resistant DTP state.
The levels of TXNRD, TXN2, PRDX5, SCL7A11, and GPX4 are increased in DTP cells. SCL7A11: Solute Carrier Family 7 member 11, SLC3A2: Solute Carrier Family 3 member 2, γGCS: γ-Glutamylcysteine Synthase, G6PD: Glucose-6-Phosphate Dehydrogenase, GSR: Glutathione-Disulfide Reductase, GSH: Glutathione, TXNRD: Thioredoxin Reductase, TXN: Thioredoxin, PRDX5: Peroxiredoxin 5, GPX4: Glutathione Peroxidase 4, ROOH: Peroxide. Glutathione peroxidase 4 (GPX4), which catalyzes the reduction of hydrogen peroxide, organic hydroperoxides, and lipid hydroperoxides, also plays a role in in the AR inhibitor-resistant DTP state. We notice that Solute Carrier Family 7 Member 11 (SLC7A11), a component of the system-c, and GPX4 are up-regulated in the DTP cells, which protect prostate cancer cells from lipid peroxidation-induced death such as ferroptosis. Involvement of GPX4 has also been observed in other therapeutic drug-resistant DTP cells [22,34]. At present, involvement of PRDX5 has only been reported in AR inhibitor-induced DTP cells. However, an elevated level of PRDX5 seems to be inversely associated with the overall survival of liver, lung, and pancreatic cancer patients, suggesting a possible role of PRDX5 in other types of cancer.
A study of AR inhibitor-tolerant persister cells may offer a new perspective on the development of CRPC, namely reversible resistance can emerge without genetic alterations of the AR signaling pathway. The DTP model seems to recapitulate, at least in part, the clinical course of CRPC where both ARpositive and negative, drug-resistant cancer cells arise. PRDX5 appears to be a useful therapeutic target for CRPC patients. It is unclear, however, whether AR-positive and negative DTP cells behave similarly towards AR inhibitor treatment, whether they are equally reversible after drug withdrawal, and whether they have similar susceptibility to PRDX5 targeting drugs. DTP cell characteristics await further investigation.
None
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Chen YQ (2024) Persister Cells: A New Perspective on the Development of Castration-Resistant Prostate Cancer?. Andrology. 13:306.
Received: 30-Jan-2024, Manuscript No. ANO-24-29370;; Editor assigned: 02-Feb-2024, Pre QC No. ANO-24-29370 (PQ); Reviewed: 16-Feb-2024, QC No. ANO-24-29370; Revised: 23-Feb-2024, Manuscript No. ANO-24-29370(R); Published: 01-Mar-2024 , DOI: 10.35248/2167-0250.24.13.306
Copyright: © 2024 Chen YQ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.