Lupus: Open Access
Open Access
ISSN: 2684-1630
ISSN: 2684-1630
Case Report - (2021)Volume 6, Issue 9
End-Stage Kidney Disease (ESKD) is managed effectively by home peritoneal dialysis in developing countries. However, long term home PD patients are faced with enormous challenges. Here we describe two non-diabetic/ anuric female patients aged 52 and 51 years who continued on Continuous Ambulatory Peritoneal Dialysis (CAPD) for 13 years and 18 years, respectively, with formidable challenges. The following are critically examined in these two patients: Ultrafiltration, Nutritional status, Blood pressure control, Neuropathy, Anaemia correction, quality of life, and management strategies.
End-stage kidney disease; CAPD; Mycobacterium tuberculous; Anaemia
Home Peritoneal dialysis is an established renal replacement therapy (RRT) in India since 1991 [1]. However, Long term home PD [CAPD]/[APD] are faced with enormous challenges due to infective and non-infective complications [2]. Despite improvement in survival, individuals on dialysis have a shorter life expectancy due to an increase in cardiovascular mortality [3]. Another effect of CAPD is a change in the peritoneal membranes function as a result of persistent exposure to glucosecontaining dialysis fluid, as well as chronic low-grade infection, which might result in increased solute transport and decreased ultrafiltration [4]. Home peritoneal dialysis survival above ten years is a rarity in India, and very few patients continue on CAPD due to various challenges and complications which include ultrafiltration issues, malnutrition, blood pressure variability, anaemia, phosphorous control, adequacy of dialysis, quality of life, electrolyte abnormalities. Here we tried to describe the clinical and laboratory characteristics of two patients who were on CAPD for more than 10 years.
Case 1 (Patient A)
A 51-year-old lady, primipara with CKD and hypertension since 2002, had been on CAPD from 2003 doing 2L × 4 exchanges of dianeal with varying concentrations. She underwent an unsuccessful live kidney transplant in 2005. Since 2003, she had experienced four episodes of peritonitis with no catheter loss (Swan-neck Tenckhoff catheter). The organisms involved include Mycobacterium Tuberculous (was treated for Mycobacterium Tuberculous (MTB) peritonitis with a four-drug regimen for 18 months), Coagulase -ve Staph aureus (twice) and Staph Warnei. She had a substantial residual renal function, with a 24-hr creatinine clearance of 50.32L and urine volume of 1100 ml/day until 2011. However, her urine output gradually declined, and she has been anuric since 2012. She also had suffered from renal osteodystrophy from secondary hyperparathyroidism with a peak intact Ipth-1456 pg/ml, Calcium 10.3 mg/dl, and Phosphorous-51 mg/dl and underwent alcohol ablation in 2014, parathyroidectomy in 2019. A computed tomography scan of the abdomen was done due to abdominal pain and variability in ultrafiltration, which showed areas of linear calcification of the peritoneum (Figure 1a).
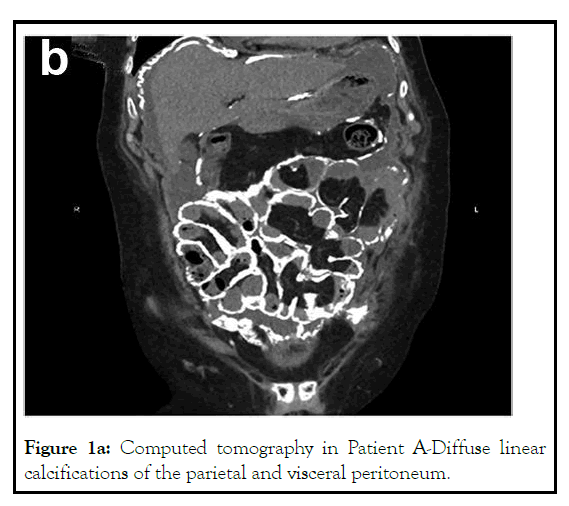
Figure 1a: Computed tomography in Patient A-Diffuse linear calcifications of the parietal and visceral peritoneum.
Case 2 (Patient B)
A 52-year-old lady, nullipara with rheumatoid arthritis since 1991 and had received parental calcium, Vitamin D supplements once a week until 2007. However, her renal function was not checked regularly. She presented to us in February 2007 with CKD. Her investigations showed normocytic normochromic anaemia with calcium 13.5 mg/dl, phosphorous 5.9 mg/dl, iPTH 6 pg/ml. A CT abdomen showed multiple dense calcified lesions in both kidneys suggestive of medullary nephrocalcinosis. Coronary artery calcium scoring showed no evidence of calcification in the coronary arteries. Subsequently, she was initiated on haemodialysis and switched over to CAPD in 2008, doing 3 × 2L exchanges of dianeal with varying concentrations. Over the next 13 years, she had experienced three episodes of peritonitis. Until 2013, she had a significant renal function, with a 24-hour creatinine clearance of 55.5L/week and a urine volume of 1500 mL/day. However, her urine output steadily declined, and she has been anuric since 2015. She also suffers from autonomic neuropathy with intermittent hypotension. She was recently admitted with a COVID-19 infection associated with abdominal pain. A chest and abdomen CT scan showed calcifications of right and left coronary arteries and linear calcification of the mid ileal loops and distal ileal loops (Figure 1b) (Tables 1 and 2).
| Biological and biochemical parameters | Patient A (51 years) | Patient B (52 years) |
|---|---|---|
| iPTH | 125.4 pg/ml | 757.6 pg/ml |
| Calcium | 9.32 mg/dl | 9.86 mg/dl |
| Phosphorous | 2.4 mg/dl | 6.21 mg/dl |
| Calcium phosphorous product | 22.368 mg/dl | 61.2306 mg/dl |
| Albumin | 2.54 g/dl | 3.05 g/dl |
| Bicarbonate | 27 mEQ/L | 28 mEQ/L |
| Haemoglobin | 8.1 g/dl | 10.8 g/dl |
| RRF | Anuric | Anuric |
| Number of exchanges | 3 × 2L exchanges of dianeal and 1 × 1L exchange of Icodextrin. | 3 × 2L exchanges of dianeal. |
| Ultrafiltration volume | Year 2005: 1100-1200 ml/day Year 2021: 850-1000 ml/day |
Year 2009: 1300-1400 ml/day Year 2021: 1000-1200 ml/day |
| Transporter status | Year 2005-low average Year 2021-High average |
Year 2009-low average Year 2021-High average |
Table 1: Distribution of biological and biochemical parameters in peritoneal dialysis patients.
|
Patient A | Patient B | ||
|---|---|---|---|---|
| Body composition analysis | Normal range in patient A | 30/012021 | Normal range in patient B | 28/01/2019 |
| ICW (Intracellular water) (L) | 16.1-19.7 | 13.8 | 20.2-24.6 | 19.8 |
| ECW (Extracellular water) (L) | 9.9-12.1 | 9.5 | 12.4-15.2 | 13.7 |
| TBW (Total body water) (L) | 26-31.8 | 23.3 | 32.6-39.8 | 33.5 |
| Protein (kg) | 7-8.6 | 6 | 8.7-10.7 | 8.5 |
| Mineral (kg) | 2.41-2.95 | 2.78 | 3.01-3.68 | 3.75 |
| Fat (kg) | 10.2-16.4 | 9.9 | 12.8-20.5 | 30.7 |
| SMM (skeletal muscle mass) (kg) | 16-19.3 | 16 | 24.6-30 | 23.8 |
| PBF (percent body fat) | 18-28 | 23.5 | 18-28 | 40.2 |
| ECW/TBW | ---- | 0.379 | ---- | 0.411 |
| Body cell mass (kg) | 23.1-28.3 | 19.8 | 28.9-35.3 | 28.3 |
| Bone mineral content | 1.98-2.42 | 2.44 | 2.48-3.04 | 3.14 |
| Waist circumference (cm) | ---- | 72.8 | ---- | 89.2 |
| BMR (basal metabolic rate) | ---- | 1064 | ---- | 1359 |
| BMI (body mass index) (kg/m2) | 18.5-23 | 17.3 | 18.5-23 | 25.1 |
Table 2: Body composition analysis of patient on CAPD.
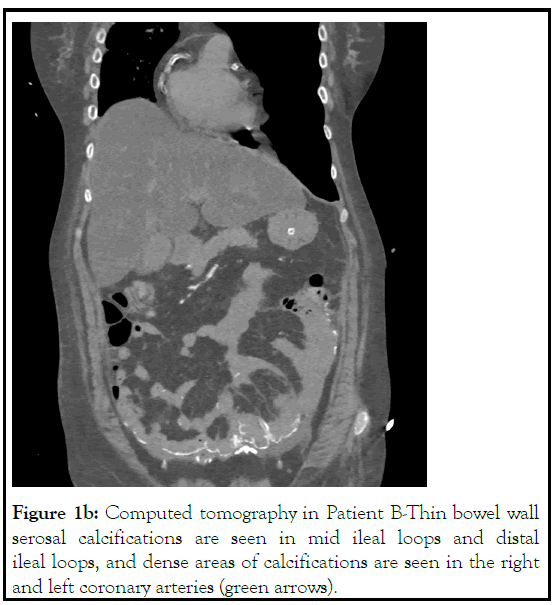
Figure 1b: Computed tomography in Patient B-Thin bowel wall serosal calcifications are seen in mid ileal loops and distal ileal loops, and dense areas of calcifications are seen in the right and left coronary arteries (green arrows).
The treatment of anuric patients on peritoneal dialysis is far more challenging. The European Automated Peritoneal Dialysis Outcomes Study (EAPOS) suggested that the main predictors of survival at the start were age, diabetes, poor nutrition and low UF volume. Survival was not influenced by small solute clearance [5]. Patients below the minimum target UF at baseline of 750 ml had a 2-year survival of 58% compared to 88% in those above the target [5]. The same observations were seen with the NECOSAD study regarding inadequate ultrafiltration and survival.
Ultrafiltration failure is defined as a volume of less than 400 mL following a four-hour dwell with 2L of 4.24% dextrose. Longitudinal and cross-sectional studies of membrane function with time on treatment have identified that glucose exposure, loss of residual renal function, and peritonitis are the drivers for the change in the peritoneal membrane dysfunction [6]. Furthermore, the study by Kolesnyk and colleagues looked at technique survival at four different periods [7]. The 1, 2, 3-year technique survivals in their study were 87%, 76% and 66% and found that ultrafiltration problems increased with increasing duration of therapy. Despite being on peritoneal dialysis for more than a decade and Mycobacterium Tuberculous peritonitis in patient A, she had good small solute clearance and recent conversion to icodextrin one exchange day along with three dianeal produced adequate ultrafiltration and solute removal (Table 1). Icodextrin was not used in patient B as a costcontainment plan as she had adequate ultrafiltration and small solute removal. Both the patients were in their thirties (patient A 32 years, patient B 37 years) when they started PD; neither developed diabetes mellitus in the course of their therapy; these characteristics appear to be beneficial to long-term membrane function as found by [7]. Both our patients were paying from their resources for the dialysis therapy. Hence, they could not afford the newer PD solutions, which may have a beneficial effect on the peritoneal structure and function.
The Hong trial study suggested that patients achieve Kt/V above 1.7 for patients with no residual renal function [8]. As patients are self-paying for PD in India, either increasing the dialysis exchanges or using peritoneum friendly dialysis solutions is fraught with economic issues. The therapy cost per month for 3 × 2L exchanges is currently Indian rupees 25732 (USD 330), and 4 × 2L exchanges a day is Indian rupees 32500 (USD 435), and Icodextrin one bag costs rupees 730 (USD 9.5). Both our patients had Kt/v between 1.7 and 1.85 during their therapy, suggesting adequacy.
Long-term exposure to dialysate, peritoneal microinflammation due to peritonitis, hyperparathyroidism can lead to extensive peritoneal calcifications [9,10]. Therefore, we suspect that secondary hyperparathyroidism; multiple episodes of peritonitis in the patients, unmonitored calcium and vitamin D3 intake before initiating dialysis in patient B may have facilitated the peritoneal calcification. In addition, these two patients are unique because of the linear peritoneal calcification. However, we are not sure the calcification observed is an early sign of encapsulated peritoneal sclerosis.
Body Composition Monitoring (BCM) studies (Table 2) showed decreased body cell mass in patient A, lower lean muscle mass in both the patients and increased percent body fat in patient B. Monitoring of BCM helped us look at hydration status as over hydration is associated with increased mortality in patients undergoing dialysis [11,12]. ESA (Erythropoiesis- stimulating agents) was used by patient A with the administration of iron sucrose since 2005 to maintain haemoglobin of ≥10 gm/dl. CKD-MBD, which leads to coronary calcification, was evident in patient B, though she had an adequate left ventricular systolic function. A previous study from India showed progressive coronary calcification with agaston score of >160 HU should be considered a marker of cardiovascular risk in dialysis patients [13]. Factors such as increased hCRP, iPTH, and longer duration on dialysis accelerate the rate of cardiac calcification. However, Patient B coronary artery calcification, as shown in the HRCT of Figure 1a, was maybe due to the unmonitored intake of calcium and Vitamin D3 earlier or progressive CKD MBD. Intermittent hypotension was observed in patient B due to autonomic neuropathy and hypovolemia, which was managed with saline infusion and midodrine hydrochloride 5 mg thrice daily. She had peripheral neuropathy of unknown aetiology, as shown by nerve conduction velocity. Both patients had good family support from spouses and other members, and the cost of dialysis could be met regularly.
Patient A engaged in agriculture and other part-time jobs, and patient B remained a homemaker throughout the course of dialysis. Although there was always apprehension about survival and social contact, dialysis staff intervention and the same nephrologists providing care helped them move on to life. The concern and care by the same nephrologists for over a decade may be an important aspect of these patients long-term survival. Patient A was immunologically sensitized and hence could not undergo kidney transplantation from her sister and brother. Patient B was content with CAPD. Though the quality of life was not measured using the various tools, both of them remained happy with their dialysis.
Patient A continuous to do well on peritoneal dialysis with a current mean ultrafiltration rate of 950 ml/day. Patient B recently passed away due to multiorgan dysfunction caused by severe COVID-19 infection burden.
Citation: Mogga P, Unny R, Kumaresan M, Kumar AN, Abraham G, Mathew M (2021) Peritoneal Calcification in Two Long Term CAPD Patients with Adequate Function. Lupus: Open Access. 6: 182.
Received: 07-Oct-2021 Accepted: 21-Oct-2021 Published: 28-Oct-2021 , DOI: 10.35248/2684-1630.21.6.182
Copyright: © 2021 Mogga P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.