Journal of Depression and Anxiety
Open Access
ISSN: 2167-1044
ISSN: 2167-1044
Research Article - (2023)Volume 12, Issue 4
Background: Depression and anxiety are complex conditions that result in significant individual disability and societal costs. Despite decades of research investigating treatment options, depression and anxiety remain a major cause of quality-of-life impairment. We hypothesized that patients with anxiety and depressive disorders might benefit from Peripheral Somatosensory Stimulation (PSS) therapy.
Methods: Ten patients with clinically diagnosed anxiety and depression were enrolled to undergo daily PSS therapy over a 4-week period. Patients were evaluated for satisfaction and overall well-being (Survey 1), severity of anxiety symptoms (Survey 2) and severity of depression symptoms (Survey 3). Survey 1 was completed at weeks 1, 2 and 4 and surveys 2 and 3 were completed as a baseline prior to initiation of therapy and then at the conclusion of the trial. All data were analyzed by an independent statistician.
Results: Ten patients were enrolled in the study; all completed the trial. There were 8 women and two men; mean age was 55.8 ± 17.1 (range 29 to 75 years). All patients demonstrated a significant time-dependent improvement in satisfaction and general well-being over the course of the trial (p<0.001). All patients reported improvement in anxiety symptoms compared to baseline and the majority described improvement in depression symptoms (p<0.01). Treatment resulted in a 19-fold higher likelihood of improvement in anxiety and a 15-fold higher likelihood of improvement in depression. No adverse events were described by the patients.
Conclusion: PSS stimulation appeared to improve symptoms in ten patients with depression and anxiety in this trial. Symptoms related to both anxiety and depression were improved significantly (p<0.01). Interestingly, anxiety symptoms improved to a slightly greater degree. We suggest that further investigation into the potential usefulness of PSS therapy in patients with anxiety and depressive disorders is warranted.
Anxiety; Depression; Neuromodulation; Somatosensory; Stimulation
PSS: Peripheral Somatosensory Stimulation; GAD: General Anxiety Disorder; PHQ: Patient Health Questionnaire; CLMM: Cumulative Link Mixed Model; IQR: Interquartile Ranges; CI: Confidence Intervals
Depression and anxiety are extremely common disorders which result in a tremendous societal burden in terms of impaired quality of life, disability, medical costs and unemployment [1,2]. It has been suggested that these conditions may be associated with impaired sensory processing, suggesting that sensory stimulation could be useful as a potential therapeutic option [3-8]. Peripheral somatosensory stimulation therapy is a non-invasive technique which may be beneficial to patients with a variety of neurological disorders. The current trial explores the use of PSS of the hand which has significant cortical sensory representation in the human brain, as a possible mechanism to improve the symptoms of anxiety and depression [9-12].
Study description
NeuroGlove is a non-invasive device that provides PSS stimulation in the form of pneumatic puffs of air directed at the volar surface of the distal forearm, the palm and the fingers. This study was designed as a prospective, single center trial enrolling ten patients to explore the effect of PSS therapy on symptoms and quality-of-life measures in patients with anxiety and depression. Men and women between the ages of 18 and 85 years with an active diagnosis of anxiety and depression who were able to provide informed consent were considered eligible for trial enrollment. Patients who were unable to comprehend or follow instructions or unable to use the device due to physical limitations of the upper extremity including fracture, joint deformity, severe spasticity/contracture or skin breakdown were excluded from participation.
Device use
Subjects were instructed to use the device at home for 1 hour of therapy per day (30 minutes using each hand) for 4 weeks. Subjects were directed to synchronize their breathing to the firing (on/off cycle) of the machine to encourage relaxation during device use. At the conclusion of the trial, compliance was determined based on patient reporting and using an internal computerized system that allowed the investigators to track device use during the course of the trial.
Patient evaluation and statistical methods
Patient response to treatment was evaluated using 3 different surveys. Survey 1 focused on patient satisfaction with the treatments and overall psychological well-being survey 2 was designed to evaluate severity of anxiety symptoms based on the General Anxiety Disorder (GAD)-7 scale (Tables 1 and 2). Survey 3 examined symptoms of depression based on the Patient Health Questionnaire-9 (PHQ) (Table 3). Patients completed survey 1 at weeks 1, 2 and 4 and completed surveys 2 and 3 at baseline and at the conclusion of the trial. Survey responses were based on a 5-pt ordinal scale. Change from baseline anxiety and depression scores were analyzed using Cumulative Link Mixed Models (CLMMs).
| Question | Description |
|---|---|
| Q1 | Neuroglove is easy to use |
| Q2 | Neuroglove helped me relax |
| Q3 | I enjoyed using the Neuroglove |
| Q4 | I would like to use Neuroglove again |
| Q5 | I would like to have a Neuroglove at home |
| Q6 | I would recommend Neuroglove to family and friends |
| Q7 | I felt more positive |
| Q8 | My anxiety/depression symptoms were improved |
Table 1: Questions from survey 1-patient satisfaction questionnaire.
| Question | Description |
|---|---|
| Q1 | Feeling nervous, anxious, or on edge |
| Q2 | Not being able to stop or control worrying |
| Q3 | Worrying too much about different things |
| Q4 | Trouble relaxing |
| Q5 | Being so restless that it is hard to sit still |
| Q6 | Becoming easily annoyed or Irritable |
| Q7 | Feeling afraid as if something awful might happen |
Table 2: Questions from survey 2 General Anxiety Disorder (GAD)-7 anxiety questionnaire.
| Question | Description |
|---|---|
| Q1 | Little interest or pleasure in doing things |
| Q2 | Feeling down, depressed or hopeless |
| Q3 | Trouble falling or staying asleep or sleeping too much |
| Q4 | Feeling tired or having little energy |
| Q5 | Poor appetite or overeating |
| Q6 | Feeling bad about yourself, or that you are a failure or have let yourself or your family down |
| Q7 | Trouble concentrating on things, such as reading the newspaper or watching TV |
| Q8 | Moving or speaking so slowly that other people could hardly notice. Or the opposite--being so fidgety or restless that you have been moving around a lot more than usual |
| Q9 | Thoughts that you would be better off dead or hurting yourself |
Table 3: Questions from survey 3 Patient Health Questionnaire (PHQ)-9 depression questionnaire.
To summarize overall severity of patient status, anxiety was considered minimal (Total scores 0-4), mild (Total scores 5-9), moderate (Total scores 10-14) or severe (Total scores 15-21). Depression was graded as minimal (Total scores 0-4), mild (Total scores 5-9), moderate (Total scores 10-14), moderately severe (Total scores 15-19) or severe (Total scores 20-27).
For survey 1, simple descriptive statistics were calculated for individual scores at each time point, including median and Interquartile Ranges (IQR). A composite score of all questions using the pooled median value of patient-specific survey questions was also generated and compared between patient visits. Additionally, for in-text summaries, scores were trichotomized as ≥ 4 (positive), 3 (neutral) and <2 (negative). For trichotomized scores, counts and percentage of total were calculated.
A CLMM was used to determine the influence of time on ordinal response scores representing user satisfaction. The main CLMM was fitted using the CLMM function with the ordinal user satisfaction scores modeled as a function of time (Week) and accounting for random effects by including a subject-specific random intercept. Laplace approximation was employed to estimate the model parameters due to its suitability for handling ordinal response data. A null model was then fitted by excluding the time variable, providing a reference against which the main model could be compared. A likelihood ratio test was then performed to assess the significance of including the time variable in the main model compared to the null model, and a two-sided p-value was extracted.
For surveys 2 and 3 changes in ordinal response scores were assessed from patient-specific matched pairs using Wilcoxon’s signed-rank test to determine if there were significant improvements from baseline to week 4. A composite score of all questions using the pooled median value of patient-specific survey questions was also generated and compared between baseline and week 4. Effect sizes from Wilcoxon’s signed rank tests were reported as the median of differences alongside approximates of the 95% confidence interval. Since this nonparametric test works with ranks, it is typically not possible to derive a confidence interval with exactly 95% confidence; instead, the closest approximate was calculated, corresponding to true Confidence Intervals (CIs) calculated with 97.85% confidence; for simplicity these are reported as 95% CIs in text.
To formally analyze overall cumulative probability of improved scores across measurement times, we employed a CLMM with a logit link function. The model was specified with the following formula using the CLMM function in the ‘ordinal’ package for R: Score ~Week+(1|Subject)
Where, ‘score’ represents individual ordinal-scale responses, ‘Visit’ is the predictor variable of interest (baseline or week 4) and ‘(1|Subject)’ indicates the inclusion of random intercepts for individual subjects to account for within-subject variability. Laplace approximation was employed to estimate the model parameters. Predicted probabilities and 95% CIs for each score at a given time point were extracted from the model. Overall effect sizes from the CLMM model are reported as cumulative odds ratios.
Simple descriptive statistics were also calculated, including median and Interquartile Range (IQRs). Additionally, for in-text summaries, scores were dichotomized as ≤ 1 (positive) or >1 (negative). Bar plots were used to visually show overall odds of improved anxiety and depression outcomes and line plots were generated to show patient-specific results across time points.
Software
All analyses were conducted in RStudio (2023.06.2 Build 561), running on R version 4.2.2. CLMM analyses were performed using the ‘ordinal’ package (version 2022.16). Figures were generated using the ‘ggplot2’ package (version 3.4.0).
Ten patients with a formal diagnosis and active symptoms of depression and anxiety were consented and enrolled in the trial. There were 8 women and two men; mean age was 55.8 ± 17.1 (range 29 to 75 years). All patients completed the trial. Compliance with device use was greater than 92% based on self-reporting and internal control checks at the conclusion of the trial. No patient reported an adverse event related to use of the device. All patients reported enjoying using the device and wished to keep the device at the conclusion of the trial. Two patients complained that the air was cool on their hand; others found the temperature relaxing.
Except for 1 patient who failed to complete survey 1 at Week 4, patients completed each of the surveys each week. At baseline, 1 patient had overall mild anxiety, 6 had moderate anxiety and 3 had severe anxiety. Regarding overall baseline depression scores, 1 had minimal depression, 3 had mild depression, 3 had moderate-severe depression, and 3 had severe depression.
Survey 1
Patient satisfaction: At last available follow-up, all patients (100%) had positive scores (scores 4 and 5) for wanting to use the device again (Q4), wanting to use the device in their home (Q5), recommending the device to family and friends (Q6), and having meaningful improvement in depression and anxiety symptoms (Q8). Regarding feelings of ease of use (Q1), relaxation (Q2) and positive feelings (Q7), 9 out 10 patients (90%) showed positive scores. Eight out of 10 (80%) patients reported enjoying using the device.
The pooled median score at week 1 was 4 (IQR: 4-4, min-max: 2-5) compared to 4 (IQR: 4-5, min-max: 3-5) at week 4, suggesting a typically positive outcome and slightly improved overall score over time (p<0.001; Table 4). The question that had the largest upward trend from week 1 (Q8) assessed improvement in depression and anxiety symptoms (Q8), showing a statistically significant improvement by week 4 (p<0.001). Other questions which exhibited time-dependent trends for improvement were those regarding relaxation (Q2), enjoying using the device (Q3) and wanting to use the device again (Q4). Other individual questions did not demonstrate statistically significant time-dependent improvements.
| Survey question | Week 1: Median Interquartile Ranges (IQR) | Week 2: Median Interquartile Ranges (IQR) | Week 4: Median Interquartile Ranges (IQR) | P-value for improvement over time |
|---|---|---|---|---|
| Q1 | 4 (4-4.25) | 4 (4-5) | 4 (4-5) | 0.843 |
| Q2 | 4 (3.5-5) | 4 (4-5) | 5 (4-5) | 0.049 |
| Q3 | 4 (3-4.25) | 4 (4-5) | 4 (3.5-5) | 0.048 |
| Q4 | 4 (3.75-4.25) | 4 (4-4.25) | 4 (4-5) | 0.032 |
| Q5 | 4 (3.75-4.25) | 4 (3.75-5) | 4 (4-5) | 0.190 |
| Q6 | 4 (4-5) | 4.5 (4-5) | 4 (4-5) | 0.162 |
| Q7 | 4 (3-4) | 4 (4-4) | 4 (4-4.5) | 0.054 |
| Q8 | 3.5 (3-4) | 4 (4-4) | 4 (4-4.5) | <0.001 |
| Overall | 4 (4-4) | 4 (4-4.25) | 4 (4-5) | <0.001 |
Table 4: Summary of survey 1 (patient satisfaction) responses across measurement times.
Survey 2
Anxiety symptoms: By week 4, all ten patients (100%) had no symptoms or mild symptoms (0 and 1) for being restless (Q5), becoming easily annoyed or irritable (Q6), and feeling afraid that something awful might happen (Q7). Regarding feelings nervousness/being on edge (Q1), inability to control worrying (Q3) and trouble relaxing (Q4), 9/10 patients (90%) reported mild to no symptoms. Eight out of 10 patients (80%) reported mild to no symptoms regarding worrying about too many different things (Q3).
Compared to baseline, patients had statistically significant improvements for all individual questions by week 4. The composite score was substantially improved from baseline, with an overall median score of 2 (IQR: 1.75-2.25) at baseline compared to 0 (IQR: 0-1) by week 4 (MD=-1.5 (95% CI: -1; -3), p=0.004; Table 5). Overall, the predicted probability of obtaining the best outcome (score=0) was 9% at baseline vs. 64% by week 4 (Table 6). Conversely, the predicted probability of obtaining the worst outcome (score=3) was 30% at baseline vs. 2% at week 4. The overall cumulative odds ratio was 19.3 (p<0.001), suggesting that on average, the odds of moving from one score to a lower (improved) score at week 4 compared to the baseline are 19.3 times higher. Overall likelihood of improved anxiety symptoms and patient-specific trends are displayed in Figure 1.
| Survey question | Baseline: Median Interquartile Ranges (IQR) | Week 4: Median Interquartile Ranges (IQR) | Median of differences (95% Confidence Intervals (CI)) |
P-value |
|---|---|---|---|---|
| Q1 | 2 (1.75-3) | 1 (0.75-1) | -1 (0; -2) | 0.016 |
| Q2 | 2 (1-3) | 0.5 (0-1) | -1 (-1; -2) | 0.004 |
| Q3 | 2 (1.75-3) | 0.5 (0-1.25) | -2 (0; -3) | 0.016 |
| Q4 | 2 (0.75-3) | 0 (0-0.25) | -1.5 (0; -3) | 0.008 |
| Q5 | 3 (0.75-3) | 0 (0-1) | -2 (0; -3) | 0.008 |
| Q6 | 2 (0.75-3) | 0 (0-0.25) | -1.5 (0; -3) | 0.008 |
| Q7 | 1 (0-2.25) | 0 (0-0.25) | -1 (0; -2) | 0.031 |
| Overall | 2 (1.75-2.25) | 0 (0-1) | -1.5 (0; -3) | 0.004 |
Table 5: Summary of survey 2 (GAD-7 anxiety) responses at baseline and at week 4.
| Survey score | Baseline probability (95% Confidence Intervals (CI)) | Week 4 probability (95% Confidence Intervals (CI)) | Cumulative odds ratio | P-value |
|---|---|---|---|---|
| Score 0 | 9% (2-15%) | 64% (49-80%) | 19.3 | <0.001 |
| Score 1 | 28% (16-39%) | 27% (16-39%) | ||
| Score 2 | 34% (22-45%) | 6% (2-11%) | ||
| Score 3 | 30% (16-45%) | 2% (0-4%) |
Table 6: Cumulative link mixed model results of overall improvement in anxiety symptoms.
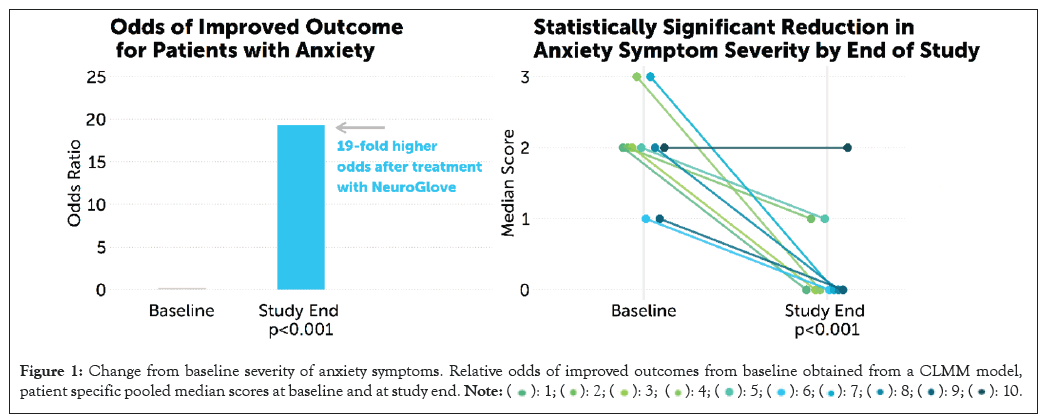
Figure 1: Change from baseline severity of anxiety symptoms. Relative odds of improved outcomes from baseline obtained from a CLMM model,
patient specific pooled median scores at baseline and at study end. 
Survey 3
Depression symptoms: By week 4, all ten patients (100%) reported being completely free symptoms (score=0) regarding moving/ speaking too slowly or being fidgety/restless (Q8). All ten patients (100%) reported positive scores (0 or 1), for little interest in activities (Q1), poor appetite or overeating (Q5), feeling bad about oneself (Q6), trouble concentrating (Q7), and thoughts about being better off dead or self-harm (Q9). Regarding feeling down, depressed, or hopeless (Q2), trouble falling/staying asleep or sleeping too much (Q3) and feeling tired or having little energy (Q4), 9/10 patients (90%) reported mild to no symptoms.
Patients had statistically significant improvements for the majority individual questions by week 4 (5/9, 67%). Although not statistically significant, patients had numerically improved scores regarding poor appetite or overeating (Q5), feeling bad about oneself (Q6), and moving/speaking too slowly or being fidgety/ restless (Q8) by week 4. For each of these questions, patients typically had mild or no symptoms, which may explain the lack of significant improvement. Regarding thoughts of being better off dead or self-harm, only 1 patient reported symptoms at baseline; this patient had improved score by week 4 (3 vs. 1), and all other patients reported no symptoms at week 4.
The composite score was substantially improved from baseline, with an overall median score of 2 (IQR: 2-3) at baseline compared to 0 (IQR: 0-0.25) by week 4 (MD=-2.0 (95% CI: 0; -3), p=0.016; Table 7), signaling typically moderate-severe depression at baseline compared vs. typically minimal to no symptoms by week 4. Overall, the predicted probability of obtaining the best outcome (score=0) was 25% at baseline vs. 83% by week 4. Conversely, the predicted probability of obtaining the worst outcome (score=3) was 31% at baseline vs. 3% at week 4 (Table 8). The overall cumulative odds ratio was 15.1 (p<0.001), suggesting that on average, the odds of moving from one score to a lower (improved) score at week 4 compared to the baseline are 15.1 times higher. Overall likelihood of improved anxiety symptoms and patient-specific trends are displayed in Figure 2.
| Survey question | Baseline median Interquartile Ranges (IQR) | Week 4 median Interquartile Ranges (IQR) | Median of differences (95% Confidence Intervals (CI)) |
P-value |
|---|---|---|---|---|
| Q1 | 1.5 (1-3) | 0 (0-1) | -1.5 (0; -3) | 0.008 |
| Q2 | 2 (1-3) | 0 (0-1) | -2 (-1; -2) | 0.004 |
| Q3 | 3 (0.75-3) | 0 (0-0) | -2.5 (0; -3) | 0.008 |
| Q4 | 3 (1.5-3) | 1 (0-1) | -2 (0; -2) | 0.016 |
| Q5 | 0.5 (0-3) | 0 (0-1) | -0.5 (0; -2) | 0.063 |
| Q6 | 1 (0-3) | 0 (0-1) | -0.5 (0; -2) | 0.063 |
| Q7 Q8 |
2 (0-3) 0.5 (0-3) |
0 (0-1) 0 (0-0) |
-2 (0; -3) -0.5 (0; -3) |
0.031 0.063 |
| Q9 | 0 (0-0) | 0 (0-0) | 0 (0; 0) | >0.999 |
| Overall | 2 (0-3) | 0 (0- 0.25) | -2 (0; -3) | 0.008 |
Table 7: Summary of survey 3 (PHQ-9 patient depression questionnaire) responses at baseline and at week 4.
| Survey score | Baseline probability | Week 4 probability | Cumulative odds ratio | P-value |
|---|---|---|---|---|
| Score 0 | 25% (7-42%) | 83% (70-97%) | 15.1 | <0.001 |
| Score 1 | 29% (19-39%) | 11% (2-20%) | ||
| Score 2 | 16% (7-25%) | 3% (0-5%) | ||
| Score 3 | 31% (11-50%) | 3% (0-6%) |
Table 8: Cumulative link mixed model results for overall improvement in depression symptoms.
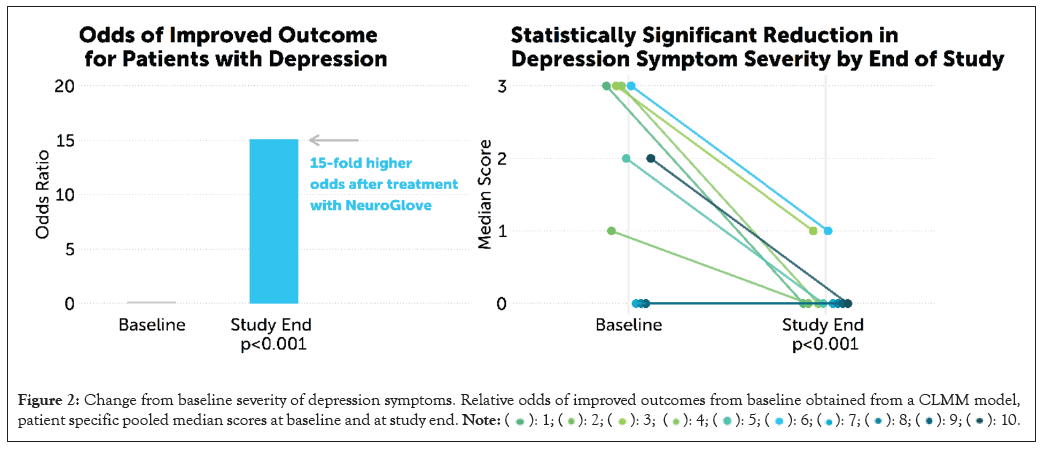
Figure 2: Change from baseline severity of depression symptoms. Relative odds of improved outcomes from baseline obtained from a CLMM model,
patient specific pooled median scores at baseline and at study end. 
A variety of physiological alterations within the brain have been associated with anxiety and depression and increasing evidence suggests that impaired sensory processing may play a critical role in these disorders [3-8]. Ayres suggested that sensory processing forms a significant basis for an individual’s physiological state [13]. Interestingly, various sensory inputs have been shown to impact symptoms in patients with depression and anxiety [14-21]. Auditory stimulation with music, olfactory stimulation with particular odors and gustatory stimulation with particular foods have all been shown to improve mood and relieve anxiety [14-17]. Transcranial magnetic stimulation and vagal nerve stimulation have also been used to ameliorate anxiety and depressive disorders [18-21]. Based on this, we hypothesized that tactile PSS might represent a straightforward therapeutic option for these conditions.
In rodent models, PSS has been shown to improve neurological outcomes following ischemic injury and to limit or even prevent stroke when applied early enough [22-24]. The mechanism for this protection remains unclear but may include enhanced neuronal reorganization encouraging neuronal recovery and/or improved regional cerebral perfusion through the recruitment of local collateral blood supply [25]. Preliminary clinical experience has suggested that PSS can improve recovery and rehabilitation in stroke survivors [26-35]. Interestingly, similar benefits have been demonstrated in patients with Parkinson’s disease, and PSS has also shown promise following traumatic brain injury and in inflammatory, auto-immune conditions such as multiple sclerosis [36-41]. Our previous work has demonstrated significant improvement in symptoms of post-traumatic stress disorder with the use of PSS [42].
In this study, we encountered a significant early response to PSS treatment as evidenced by the improvement in symptoms just one week after initiating therapy. This benefit appeared to be sustained and to further increase over the course of the study. Interestingly, multiple patients reported that PSS helped them relax and spontaneously noted an improvement in their ability to fall and remain asleep, a parameter we had not originally included in our assessment.
In regard to satisfaction with the treatment and overall sense of well-being, patients demonstrated the greatest improvement regarding depression/anxiety symptoms, relaxation, enjoying using the device and wanting to use the device again. The pooled median scores on survey 1 demonstrated significant and time-dependent overall improvement. No patients demonstrated negative survey responses by the week 4 survey.
In regard to anxiety symptoms, patients demonstrated unanimous improvement in anxiety symptoms by week 4, with all individual questions demonstrating significant improvement and composite scores substantially improved from baseline. Overall results demonstrated that patients had moderate-severe anxiety symptoms at baseline compared to minimal or no symptoms by week 4. Patients demonstrated unanimous improvement in depression symptoms by week 4, with the majority of individual questions demonstrating significant improvement and composite scores substantially improved from baseline. Results from survey 3 suggest that, on average, patients typically had moderate-severe depression symptoms at baseline compared to minimal or no symptoms by week 4. It is interesting to note that anxiety symptoms improved to a slightly greater degree than depressive symptoms, although patients reported significant improvement in both areas over the course of the trial.
We describe the results of a clinical trial evaluating the impact of one month of treatment with PSS on symptoms in patients with a diagnosis of depression and anxiety. All patients completed the trial and all appeared to benefit from the therapy. The improvement in symptomatology was generally apparent at one week of device use and was sustained and typically increased through the course of the trial. A significant reduction in both anxiety and depression symptoms was achieved when comparing baseline (pre-trial) and 4-week (post-trial) assessment using the GAD-7 and PHQ-9 scales. We suggest that further investigation into the potential use of PSS in the treatment of patients with depression and anxiety is warranted.
The main limitations of our study are the small sample size and lack of an active control group. Data are also limited only to self- reported survey questions and may not capture other clinically important outcomes. Nevertheless, this trial was meant to evaluate in preliminary fashion the potential usefulness of PSS in the treatment of patients with anxiety and depression, potentially forming the basis for a larger study.
Drs. Leslie and Eric Nussbaum are shareholders in NeuroGlove, LLC. The author declares that there has been no conflict of interests with any individual or organization in the conduct of this study and further declares that no funding has been received from anywhere for this study.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Nussbaum LA, Janjua TM, Pederson JM, Nussbaum ES (2023) Peripheral Somatosensory Stimulation in the Treatment of Depression and Anxiety: A Clinical Trial. J Dep Anxiety. 12:525.
Received: 29-Nov-2023, Manuscript No. JDA-23-28263; Editor assigned: 01-Dec-2023, Pre QC No. JDA-23-28263 (PQ); Reviewed: 15-Dec-2023, QC No. JDA-23-28263; Revised: 22-Dec-2023, Manuscript No. JDA-23-28263 (R); Published: 29-Dec-2023 , DOI: 10.35248/2167-1044.23.12.525
Copyright: © 2023 Nussbaum LA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.