Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Review Article - (2025)Volume 16, Issue 9
Percutaneous revascularization of ostial circumflex artery (LCx) lesions is known to be associated with suboptimal results. Evidence about the optimal treatment strategy for these lesions is limited. This review focuses on the main issues with coronary revascularization of these lesions and analyses the available evidence in this setting
Coronary lesions at the ostium of epicardial vessels have a high elastic fiber and calcium content, which may increase elastic recoil during the intervention, increase the rigidity of the vessel wall and reduce vessel distensibility [1]. Furthermore, stent positioning must be accurate for adequate ostial coverage and carries the additional risk of plaque or carina shift if the lesion is located at the Ostial Anterior Descending artery (LAD) or Left Circumflex (LCx).
A steep angulation of the LCx take-off may lead to significant shear stress and increased atherosclerosis development, thus high event rates after stenting. A stent crossing over from the Left Main Coronary Artery (LMCA) to LCx is subjected to torsion, flexion and rotational forces which may lead to stent fatigue and fracture, thus prompting restenosis. Likewise, larger bifurcation angle changes during the cardiac cycle have been associated with higher rates of Target Lesion Revascularization (TLR) [2].
As such, Percutaneous Coronary Intervention (PCI) of ostial LCx lesions is associated with suboptimal results. This review will go through the main issues with this treatment (Figure 1) and the available PCI strategies.
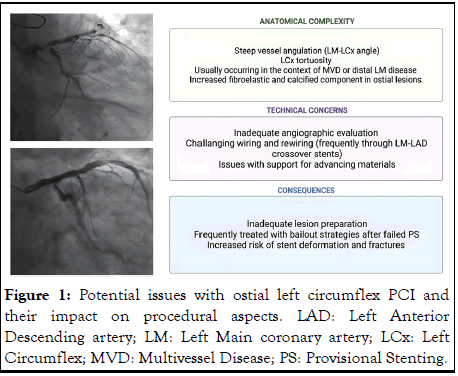
Figure 1: Potential issues with ostial left circumflex PCI and their impact on procedural aspects. LAD: Left Anterior Descending artery; LM: Left Main coronary artery; LCx: Left Circumflex; MVD: Multivessel Disease; PS: Provisional Stenting.
Who are the patients with an ostial circumflex lesion undergoing PCI?
Previous studies showed that patients with severe ostial LCx lesions have a high prevalence of comorbid conditions, including diabetes (32%-42.2%), prior myocardial infarction (28.2%-46.8%), chronic kidney disease (17.4%-26.1%) [3]. Multivessel disease (70.2%-94.2%) and a prior myocardial revascularization (coronary artery bypass grafting, CABG, 23%-32% or PCI 36.2%-49.7%) are common. Medical treatment is frequently chosen as a first choice (up to 63% of the patients) and ostial LCx PCI is then performed after symptoms recurrence or in case of residual ischemia [4].
Such a demographics may account for poorer outcomes after PCI. Higher mortality and Major Adverse Cardiovascular Event (MACE) rates were reported for patients undergoing ostial LCx PCI if compared to ostial LAD PCI (MACE rate 16.7-20 vs. 12.5%; all-cause death 7.7-11.2% vs. 8.4% at 1 year).
Ostial LCx disease may be seen as a marker of elevated atherosclerotic burden as much as it is a risk factor for further events.
Which strategy to use for ostial circumflex lesions?
Different stenting strategies are available for the treatment of ostial LCx (Figure 2). Dual stenting is usually needed when a severe ostial LCx disease is present in the context of a Medina 1,1,1 disease involving the ostial LCx. While an upfront two stent strategy using DK-crush or Culotte technique is usually the strategy of choice when the lesion is more extensive, a T and Protrusion (TAP) and T-stenting technique is frequently used as a bailout when a provisional strategy fails.
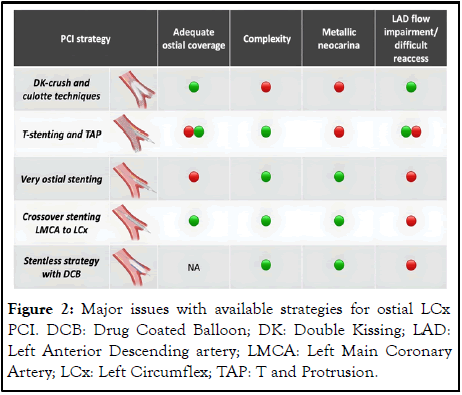
Figure 2: Major issues with available strategies for ostial LCx PCI. DCB: Drug Coated Balloon; DK: Double Kissing; LAD: Left Anterior Descending artery; LMCA: Left Main Coronary Artery; LCx: Left Circumflex; TAP: T and Protrusion.
In case of isolated disease of ostial LCx (Medina 0,0,1) additional strategies available are crossover stenting from the LMCA to LCx and spot stenting at the level of ostial LCx. These strategies raise concerns about compromising the main branch and re-accessing to the LAD.
Disease involving the LM and LCx ostium (Medina 0,1,1) can be either treated with un upfront two-stent strategy or with a crossover strategy from the LM to the LCx. The second strategy is rarely used as a default one, while it is commonly used if the LAD is occluded or protected by a bypass graft. A recent study from our group comparing the available strategies for the treatment of ostial LCx lesions showed no statistically significative difference in event rate after very ostial stenting, crossover stenting from LMCA to LCx or a two stent strategy in terms of target vessel revascularization (TVR at 2 years respectively in 17.7%, 13.8% and 22.5% of the patients) and major adverse cardiovascular and cerebrovascular events at two years (MACCE at 2 years respectively in 27.6%, 22.9% and 34.7%).
Evidence from previous trials
Multiple previous trials addressed the issue of optimal stenting strategy for the LMCA bifurcation. The comparison of Double Kissing Crush versus Culotte stenting for unprotected distal left main bifurcation lesions (DKCRUSH III) trial showed an higher mace rate with Culotte stenting than with DK-crush (16.3% vs. 6.2%, p=0.001), mainly because of significantly increased TLR and TVR in the Culotte group (respectively 2,4% vs. 6.7% and 4.3 vs. 11%) [5]. Notably, at 1 year follow-up angiographic restenosis was more frequently located at the ostium of the LCx in both groups (5.1% with DK-crush vs. 9.2% with Culotte, p=0.045).
In the randomized study on Double Kissing Crush technique versus provisional stenting technique for coronary artery bifurcation lesions (DKCRUSH-V), the incidence of TLR was lower with DK-crush than with provisional stenting, respectively occurring in 5% vs. 10.7% of patients at 1 year (HR 0.42 (95% CI 0.21-0.85), p=0.02) [6]. Angiographic restenosis was more frequently located at ostial LCx (12.0% with provisional stenting and 5.0% with DK-crush, p=0.09).
In the European bifurcation club left main coronary stent study (EBC MAIN) trial an equivalent mace rate was observed in patients randomized to a stepwise provisional stenting or systematic dual stenting (respectively, 14.7% and 17.7%, p=0.34) [7]. Most dual-stent procedures were Culotte (53%) or T/TAP (33%).
Challenges with DK-crush and Culotte techniques
DK-crush, mini-crush and Culotte techniques are the two-stent strategies of choice for bifurcations with a relevant involvement of the Side Branch (SB), unless the bifurcation angle approximates 90°. However, they are complex techniques comprising multiple steps and re-wiring through stent struts.
Previous bench tests and tomographic imaging findings reported that a “napkin” or a gap or a metallic ridge is usually seen at the ostial SB after Culotte stenting, leading to the failure to fully cover the ostial SB and resulting in increased in-stent restenosis and TLR [8].
For DK-crush, the presence of stent malapposition or geographic miss and the position of SB rewiring before Kissing Balloon Inflation (KBI) potentially have an influence on clinical outcomes.
Challenges with T-stenting, TAP and V stenting
T-stenting and TAP techniques are a limited complexity upfront two-stent techniques for bifurcations with angulations close to 90°, especially when the SB is consistently smaller than the main branch.
Besides being easier and faster to perform than DK crush or culotte, these techniques have the great advantage of representing a potential bailout in case of inadequate results on the SB after provisional stenting of the main vessel.
While the TAP technique grants adequate SB ostium coverage, a neocarina is created from the SB stent struts that may impede equipment advancement in the main vessel. Moreover, when the TAP technique is used in narrow angulation, various degrees of SB stent protrusion into the MB are necessary, creating a single layer neocarina.
V stenting can be an option for Medina 0,1,1 bifurcation. It is a simple technique, with no need for rewiring or for kissing balloon inflation and always allowing the maintenance of wire access in both MV and SB. The main disadvantage is the formation of a neocarina that can hinder subsequent wiring and equipment advancement.
The role of very ostial stenting
Very ostial LCx stenting may be chosen if an isolated ostial LCx disease is present. Challenges exist with adequate stent positioning. Excessive stent protrusion in the LMCA may impair the flow or hinder subsequent treatment of the LAD. Inadequate coverage of the ostium results in increased risk of instent restenosis. Musallam, et al. showed a 16.7% 1-year MACE rate for this strategy, while a Target Lesion Failure (TLF) rate of 24.5% at 3 years was reported by Watanabe, et al. notably, 12.5% of the these patients required LMCA-LAD PCI after ostial LCX stenting [9].
Crossover stenting from the LMCA to LCx
Crossover stenting from the LMCA to LCx is rarely used for LMCA bifurcation lesions due to concerns with compromising ostial LAD. This strategy was reported to be associated with a high rate of TLR for both the LCx ostium and LAD ostium in a small population of patients with unprotected LM disease mostly treated with first generation DES by Naganuma, et al. [10].
Stentless strategy using Drug Coated Balloon (DCB)
Given the limitations with PCI with stents at the level of ostial LCx, a stentless strategy seems appealing. Recently, increasing evidence have been built about the performances and clinical outcomes with DCBs in de novo lesions.
The published experience reports the use of DCB to be associated with less angiographic late lumen loss and similar rates of restenosis and revascularization in comparison to DES. Experience with ostial lesions was reported by Lu, et al. with 25.83% of lesions involving ostial LCx [11]. A hybrid DES and DCB or DCB only strategy (58/120 patients) led to a TLR rate of 5.26% at 24 months follow-up and a mace rate of 7.89%.
Strategies for optimizing ostial lesions PCI
Lesion preparation: Superimposed calcification and angulation at ostial LCx, pose peculiar challenges for optimizing lesion preparation. Stent underexpansion due to insufficient lesion preparation and inadequate post-dilatation may increase the risk of TLF. Rotational atherectomy is underused due to concerns about an increased risk of vessel perforation and dissection that may compromise blood flow to a large myocardial area. Tips for performing rotational atherectomy for ostial LCx lesions are the following [12]:
•Use a supportive guiding catheter, coaxial to the LCx lesions (mainly extra-back up, EBU or contralateral left support, CLS)
•Be sure to have a good backup from the Rotawire by wiring to the distal end of the vessel
•A 1.5 mm burr can be an advantageous initial burr choice (a larger burr may need a greater ablation time and be more traumatic on the vessel, while a smaller burr may be at increased risk of burr entrapment)
•A small balloon pre-dilatation may make further ablation easier.
Tortuosity of the LCx is a further challenge, as it impedes adequate angiographic assessment with standard projections and a smooth wiring using standard workhorse guidewires, plus it raises issues with the lack of support for advancing materials in the vessel.
The role of intracoronary imaging
Intravascular imaging is associated with improved outcomes for LMCA PCI. It comprises three steps:
Pre-procedural assessment: Evaluation of the reference vessel diameters, minimal lumen diameter, minimal lumen area, plaque burden, plaque morphology. This allows appropriate stent sizing and lending zone evaluation, while aiding lesion preparation.
Guidance during PCI procedure: Intravascular imaging can detect guidewire position, stent struts apposition and confirm full SB crushing in DK-crush.
Post-PCI assessment: Stent optimization implies adequate stent expansion, stent symmetry and no stent malapposition. Stent underexpansion is the most important mechanism of DES failure with a Minimal Stent Area (MSA) of less than 5.0 mm2-5.5 mm2 as the best IVUS predictor for first generation DES restenosis [13]. Optimal MSA cutoffs after PCI are 5.0 mm2 for the LCX ostium, 6.3 mm2 for the LAD ostium, 7.2 mm2 for the polygon of confluence (the common origin of LAD and LCx) [14]. As such, selecting stents with greater internal diameter may improve clinical outcomes after PCI. The specific stent platform characteristics should also be considered, especially the stent upper overexpansion limit, to avoid stent fractures with postdilatation, which is an established risk factor for restenosis.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Cozzi O, Maurina M, Reimers B, Regazzoli D (2025) Percutaneous Coronary Intervention for Ostial Circumflex Lesions: Challenges and Available Evidence. J Clin Exp Cardiolog. 16:973.
Received: 24-Dec-2023, Manuscript No. JCEC-23-28649 ; Editor assigned: 26-Dec-2023, Pre QC No. JCEC-23-28649 (PQ); Reviewed: 09-Jan-2024, QC No. JCEC-23-28649 ; Revised: 09-Jan-2025, Manuscript No. JCEC-23-28649 (R); Published: 16-Jan-2025 , DOI: 10.35248/2155-9880.25.16.973
Copyright: © 2025 Cozzi O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.