Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Research Article - (2020)Volume 11, Issue 1
Manipulating the cell’s inherent vulnerabilities to induce intrinsic changes and re-engineer diseased tissues to
drive regeneration is a domain creating significant therapeutic impact through translational medicine. Articular
cartilage injuries are recurrently experienced due to trauma, mechanical stress and age-related deterioration.
Prevailing mend methods have failed to yield reliable or lasting results. Lack of suitable models that mimic the
cellular and extra cellular matrix properties of hyaline cartilage has been one of the reasons for this deficiency.
We investigated and observed, joint tissue obtained during total knee replacement surgeries from 7 discrete
patients presenting with osteoarthritis and the commonly associated symptoms of inflammation and pain. We used
modulated “Nuclear Magnetic Coupled Fast Radio Burst or simply Fast Radio Bursts”, a new modality that seems
to trigger the cellular signaling required by the articular cartilage tissue, to mend and regenerate by upregulation
of Heat Shock Protein 70. Fast Radio Bursts are high energy, short electromagnetic bursts, in which both electric
and magnetic components of the electromagnetic signals are “circularly” polarized. Fast Radio Bursts are
produced when a radio signal is traveling through a powerful instantaneous magnetic field on its path to the target.
In this Sham controlled study, up-regulation of Hsp 70 protein, to establish its role in an in vitro model was
designed to expose 2-Dimensional and 3-Dimensional cultures to Fast Radio Bursts, and compared to a Sham
Control, under identical conditions but without exposing Sham Control culture to fast radio bursts. The 2D and
3D reconstructed cartilage tissues were then assessed in both groups. Experiments were conducted to characterize
the 2D and 3D cultures to confirm total collagen, fibrillar collagen and proteoglycans, additionally;
immunofluorescence and cell viability assay was performed to identify specific bio markers like Collagen 1,
Collagen II, Aggrecan, Cell surface Adhesion factor, Hsp70 and cell viability. It could be established in this study
that 2D cultures grown in newly defined media and exposed to Fast Radio Burst signals, when compared to 2D
cultures grown as Sham culture showed more chondrocyte specific markers and viable matrix properties. In 3D
cultures grown similarly also showed better deep layer properties compared to the 3D cultures grown in sham
culture. Thus modulated Fast Radio Bursts exposure could play a significant role in specific protein up/down
regulation in tissue regeneration.
Osteoarthritis; Articular cartilage; Hyaline cartilage; Heat shock protein 70; Nuclear magnetic coupled fast radio burst
Articular cartilage (AC) is a conformation of hyaline cartilage (HC) which is primarily controlled by the extracellular matrix (ECM) rich in collagen II and proteoglycans (PG) like aggrecan. It has been described previously in the literature that the AC tissue has a low level of regenerative capacity with a bare 5% chance of repair, due to mechanical stress bearing structures being found in layers of HC [1-3]. The HC formed in five different layers with different amounts of collagen, proteoglycans and cellular content was found to be abundantly present in the deep layer of native AC [4-6]. Among all the layers, the deep layer (L-III) of the native AC is generally the most stress resistant as it comprises thick collagen fibrils, high proteoglycan content and decreased water concentration [7-9]. This structural organization can be damaged due to mechanical stress such as excessive exercise or sports related trauma or other activities that lead to osteoarthritis and arthritis [10-12]. Certain new cell based technologies have been implemented for the management of these conditions, such as matrix assisted and autologous chondrocyte implantation, autologous mesenchymal stem cell transplantation etc. Micro-fractures are also sometimes induced to enhance the pain and stop the degradation of the joint tissue [13-15]. New strategies are currently being developed for manufacturing the scaffold-based tissue engineering materials by utilizing natural materials and synthetic, bio molecules, growth factors, ECM and genetically modified cells that could aid in repairing of the degraded AC [16-19]. We selected the collagen type I gel as a natural bio material that is highly bio- compatible and absorbable. To improve on the prevailing strategies for articular cartilage repair, a better study of the depth and grade of joint damage is necessary, so that the newly regenerated tissue is without defect and replicates the cellular and extracellular matrix properties of native AC [20]. Newly Defined Medium (NDM) enriched with human umbilical cord serum (UCS) [21,22], has been described as a better alternative than fetal bovine serum (FBS) commonly used to culture mesenchymal cells. We have extensively compared 3D cultures in two different groups; the Sham Control (SC) group and the experimental group exposed to Nuclear Magnetic coupled Fast Radio Burst (NMcFRB). Molecular properties of these cultured chondrocytes and reconstructed 3D cartilage with native AC properties was also studied. Our results distinctly showed that three dimension cartilages was reconstructed by using the NMcFRB exposure to chondrocytes grown in NDM, yields more cellular and ECM properties that are closer to those of the deep layer of HC. Pooled UCS was obtained from 16 independent volunteer umbilical cord blood donors during Cesarean section from Raja Rajeswari Medical College and Hospital. In the cartilage tissue obtained during Total Knee Replacement (TKR) surgery, it was observed that the AC is damaged due to mechanical stress and age related deterioration that led to osteoarthritis, arthritis and rheumatoid arthritis. The commonly available orthopedic solutions using prosthetic replacements with titanium, or magnesium alloy in the injured joint have allowed somewhat significant improvements that are far from becoming clinically satisfactory advancements in alleviating the pain suffered by affected individuals. We can repair the articular cartilage at the injured site by externally growing, reconstructing and implanting the autologous articular cartilage into the injured site or possibly expose the injured joint directly to nuclear magnetic coupled fast radio burst for in situ reconstruction. Correcting the protein structure which has been misfolded leading to arthritis, osteoarthritis and related co-morbidities might be a good solution. Establishing that this is technically possible by up-regulating the Hsp70 family signaling pathway to prevent chondrocytes from deterioration using non-invasive NMcFRB, would change the present orthopedic practices to improve and provide a better quality of life of affected patients and individuals and may extend the need for total knee replacement surgery.
Existing evidence has illustrated how the protein structure can be corrected by different means of molecular interplay and mimicry through the heat shock protein cellular signaling pathway, indirectly verifying our hypothesis. Proper folding and assembly of poly peptides depends on a set of conserved proteins known as molecular chaperons. Although many chaperons are classified as stress induced proteins, they also perform essential functions under normal physiological conditions. Some of these proteins temporarily stabilize the unfolding of partially folded proteins and thus promote the generation of the correct tertiary structure. To name a few, the most important proteins like Hsp 40, Hsp 60, Hsp 70 and Hsp 90 play independent roles in this complex protein structure. Many molecular chaperons described to date are members of the Hsp60 and Hsp70 families. Hsp70 cognate proteins in the cytosol associate with newly formed poly peptides during ribosomal translocation and are directly involved in protein transport processes between different intracellular compartments that lie across the membrane. In eukaryotes, one of the Hsp70s (Hsp73) participates in the lysosomal degradation of cytosolic proteins while another Hsp70 cognate protein plays an essential role in protein translocation into the endoplasmic reticulum (ER) [23]. Mitochondrial Hsp 70, for example, promotes protein translocation into the mitochondria. It is required for subsequent folding of newly translocated proteins. This feedback loop responds to any increase in misfolded proteins (such as those produced by elevated temperature) by boosting the chaperons that help the proteins to refold [24] (Figure 1).
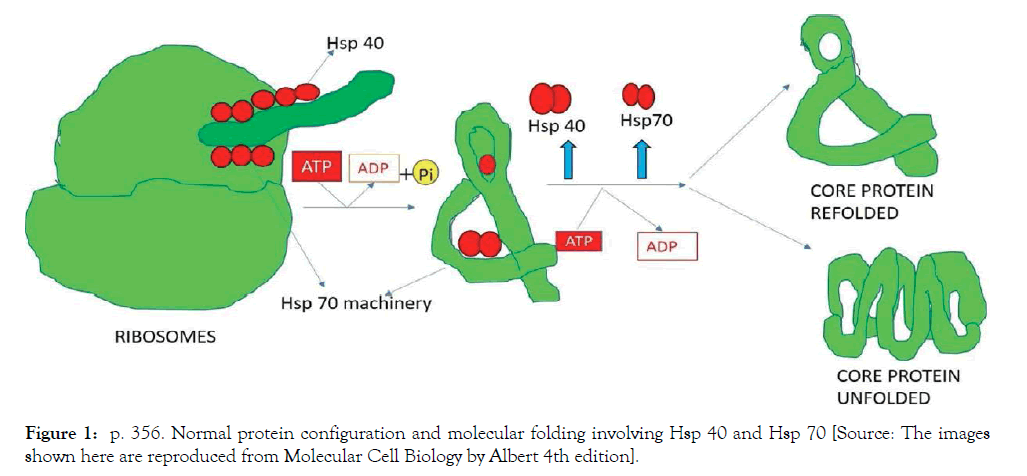
Figure 1: p. 356. Normal protein configuration and molecular folding involving Hsp 40 and Hsp 70 [Source: The images shown here are reproduced from Molecular Cell Biology by Albert 4th edition].
Mitochondria contain their own molecules such as Hsp 40 and Hsp 70 which are noticeable from those that function in the cytosol, particularly Hsp 70 that helps in folding the proteins in the endoplasmic reticulum. As indicated, these proteins act early by recognizing a small stretch of hydrophobic amino acids on its surface, supported by a set of smaller Hsp 40 proteins and Hsp 70 monomers that bind to its target proteins to induce functional molecular change [25].
The initiation of osteoarthritis (OA) is characterized by severe inflammation, when biological systems increase the heat shock proteins, particularly Hsp70, the most inducible stress protein. The anti-apoptotic activities exerted by hsp70 is by inhibiting pro-inflammatory synthesis or factors that will lead to apoptosis and inflammatory pathways, as activation of caspases; JNK signaling pathway that releases cytochrome C; and the formation of the apoptosome, which is a hallmark of apoptosis and progressive inflammation [26]. More so, it is surprising that Hsp70 is up-regulated in the synovial membrane during OA and modulates the effects of the T-cells while controlling inflammation via the inhibition of pro-inflammation signals [27]. In consistent findings, several studies have shown T-cell response to Hsp70 through production of interleukin 4 and interleukin 10. These regulatory cytokines suppress arthritic diseases in many animal models. These were observations suggested to cross the reactivity of Hsp with T-cells may be one of the ways of managing human inflammatory diseases like osteoarthritis and rheumatoid arthritis under stressed physiological states [28].
It is hypothesized that NMcFRB is able to promote cell regeneration by cellular reprogramming by interaction with the proton density (PD) or density of hydrogen nuclei in the chondrocyte. Exposing human chondrocyte cells derived from articular cartilage tissue which is present in the hyaline cartilage to NMcFRB has shown by objective evidence of the degree of regeneration that has occurred to a specific injured part of the AC tissue in vivo [29]. Such a mechanism of action can be proven by exposing the human chondrocyte cells that are derived from a patient suffering from osteoarthritis to NMcFRB, and provide a biomolecular evidence for the possible repair of osteoarthritis, arthritis and other chondrogenesis disorders in vitro. This is based on the hypothesis that NMcFRB can ‘recognize’ AC tissue damage by changing the PD and accordingly alter the Transmembrane potential (TMP) related pathways, which in turn would up regulate the Hsp 70 protein activity and prevent the damaged AC from further deterioration. The development of osteoarthritis is in large part driven by inflammatory processes. Therefore, the studies have proposed that regenerated protein structure is partially responsible for the positive outcomes in clinical osteoarthritis trials. This is in contrast to prevail in literature about cartilage-related TMP alteration [29]. A fast radio burst (FRB) are radio waves that are signal polarized or in simple terms means that the radio wave is being warped, as they travel in space between radio emitting antennae and the target, as the waves are subjected to high instantaneous magnetic fields. Radio waves that pass through the magnetic field are twisted in a process known as ‘Faraday rotation’. The stronger the instantaneous magnetic field, the greater this twist. It is believed that FRBs are produced in space, and they reach earth on regular intervals. Radio waves produced by space objects become FRBs as they travel through high magnetic fields in their travel path, illustrated in Figure 2. Extraterrestrial origin of FRBs and its effect on living cells are not well understood [30]. Many recent studies have shown that living cells can indeed be reprogrammed, to alter itself, de-differentiate and re-differentiate [31,32] This is however not part of or the scope of this study.

Figure 2: Fast radio burst as they are conceptualized in nature.
The NMcFRB is produced by a machine specially designed and delivered by the Centre for Advanced Research and Development (CARD), Bangalore campus, upon our request. It is a small circular gantry machine that can be placed inside an incubator. It has four helical Radio frequency (RF) antennae and powerful instantaneous magnetic guns. The radio frequency produced is in the 68 MHz band modulated with desired bursts signals (BS). The magnetic guns aligned along the radio frequency signals can deliver very high instantaneous magnetic fields to cause the necessary polarization in the modulated radio waves to produce Fast Radio Bursts. The interactions between Radio frequency radiation and living matter is now becoming an important area of research; even though these phenomenon has been observe red since the early times. Many experimental evidences today shows that modulation of biological functions are possible to be controlled by application of different types of Radio frequency Radiation in the electromagnetic spectrum. Generally available technologies for detection all measurements of endogenous electrical and Magnetic variations in living organisms and their components, has encouraged further, exploration in to its biological effects.
There are three types of broad band electromagnetic radiation-induced effects on living matter; Ionizing effects (x-rays, radiotherapy), thermal effects (as in RF ablation), microwaves, non-ionizing effects (as in Electromagnetic fields at the lower end of the electromagnetic spectrum, however some amount of thermal effect is also seen in biological systems). Fast Radio Bursts are purely non-ionizing and non-thermal (as in IMcFRB). Ionizing effects knock off electrons from the atoms in the molecules, thus causing uncontrolled destruction. Radio thermal effects (like microwaves) induce an entropic disorder in the target tissue or cell those levels to hyperthermic destruction in its target. The non-thermal, non-ionizing effects are the result of the transfer of ionic movement by means of an increase in kinetic energy, coherence line with theories of condensed matter. This kind of radio signals can transmit infrastructure specific to wave form, string of waves, the time sequence of their modulation pattern, spin around the target, rotation, power density of Fast Radio Burst signaling and delivery. In fact, the configuration and temporal exposure patterns of NMcFRB can produce highly specific biological responses, similar to many powerful chemical and pharmaceutical interventions.
Processes such as FRB portrayed in Figure 2 are attracting considerable scientific interest mainly because radio frequency beams can be easily modulated and are an excellent means for the transmission of well-coded information. The FRB’s can be generated and used to manipulate and reprogram precise cellular command and control. The internal and external interactions of biological cells can be modeled in terms of its electric, magnetic and electromagnetic relationship. Studies have shown that it is possible to alter and control the selective permeability of cell membrane by exposure to spatially polarizing and rotating FRB Quantized packets [33]. This makes it possible to verify the specific reactions of healthy cells, compared to reactions of pathological cells and subsequently to selected target cells to manipulate for clinical purposes. Diseased cells are different from healthy cells due to different tissue composition that affects its proton density, permittivity, permeability, conductivity etc.
Nuclear magnetic coupled Fast Radio Burst is produced by a device that is designed to deliver highly complex, quantized, instantaneous polarized beams in radio frequency bands and its harmonics ranging from 3 to 300 MHz experimental device shown in Figure 3.

Figure 3: NMcFRB machine for this study: The gantry that delivers the NMcFRB described in the methodology is possible with this specialized device constructed for the purpose. It is designed to hold cell culture plates not exposed in the sham control or exposed in the experimental procedure, and can be place within a CO2 incubator shown below.
This is done by allowing the radio beams to travel through a high instantaneous magnetic field between 1mT to 6T lasting for a few microseconds, with near field delivery using specialized delivery guns (K- Ferrite type: Near Field: gain: 10 dB). The radio beams are delivered through special antenna (Helical, Qfactor 560, Gain 13 dB, center frequency 68.4 MHz, 3 dB band width ±4 MHz, 6 dB band width ± 10 MHz) [34]. It is hypothesized that when these beams are appropriately controlled and modulated, they can alter presumptive protein pathways to meet specific requirements like stimulating the up-regulation of Hsp 70/90 group protein synthesis in AC regeneration. The delivered instantaneous mode magnetic and RF modulation in the form of NMcFRB is highly cell specific. This manipulation of the cell membrane potential in vitro will alter specific proteins required for tissue regeneration, which is integral to NMcFRB.
With this hypothesis that chondrogenesis can respond to FRB, the NMcFRB can be precisely controlled and focused on cells/tissues in culture (in vivo), using fixed proton density (PD) dosimetry method. Proton density or the density of the hydrogen nuclei (H+) in the chondrocytes are measured using Nuclear Magnetic Resonance (NMR). FRB generated streaming potentials flow in the cells and cause forced movement of protons in the extracellular matrix (ECM). This is caused by alteration in the spin of the hydrogen protons and subsequent nuclear realignment, leading to a decrease in the transmembrane Potential (TMP) of the cell.
This is a triggering factor inducing the Hsp 70 group of proteins in terminally differentiated chondrocytes, chondroblasts and chondroprogenitor cells, resulting in stimulating the upregulation of the required Hsp70 and other proteins in this group to initiate cell division or mitosis [35,36]. Transmembrane potential or TMP, one of the key protein signaling pathways, is the electrical potential difference between the exterior and interior of the biological cell.
In relation to the exterior of the normal cells, typical values of membrane potential range between -40mV to -90mV. Virtually all eukaryotic cells maintain a non-zero transmembrane potential, which contains a negative voltage in the cells interiorly, as compared to the cell exteriorly, typically ranging from -40mV to -90mV. In both excitable cells and in non-excitable cells, in their baseline states, the transmembrane membrane potential is held at relatively stable values, called the resting potential [37,38].
Materials
Cell culture media: Newly Defined Medium (NDM) is prepared as follows: 1x L-Glutamine, 1x penstrep (both from Gibco), Dulbecco Modified Eagle Medium (DMEM High Glucose) and 10% human UCS. This serum was prepared from human umbilical cord blood acquired from voluntary donors from Rajarajeswari Medical College and Hospital, Bangalore, India.
Other reagents: Collagen type I gel with (cat no: 3447-020-01; Cultrex); sterile Dulbecco phosphate buffered saline (PBS), free of Ca+ and Mg2+ 0.25% trypsin (vol/vol).
Antibodies: Rabbit polyclonal to collagen I (ab 34710), collagen II (ab34712), CD44 (ab 51037), agg (ab 36861), Hsp 70 (ab 45133), Alexa Flour 594 goat ant rabbit IgG (ab150080). All the primary as well as the secondary antibodies used were purchased from Abcam Products, and Fluoroshield with 4,6-diamidino phenylindole (DAPI) (sigma product).
Surgical equipment and plastic ware: Equipment consisted of scalpels, sterile forceps, sterile scissors and sterile blade no. 7, 35 mm petri dishes, T-25 culture flask, 6-24 well cell culture inserts (Cat No: CLS3470 and CLS3472 respectively; Costar).
Methods
Collection of the human articular cartilage tissue: The visibly of good part of the articular cartilage from the medical waste during TKR was obtained under sterile conditions from Osteoarthritis and other arthritic knee joints of patients who were operated. All requirements of the Institutional Ethics Committee of Raja Rajeswari Medical Hospital and College, Mysore road, India was preobtained. The study was conducted with seven samples collected over a period of one year. The study was also approved by the Institutional Committee for Stem Cell Research (ICSCR) at Manipal Institute of Regenerative Medicine (MIRM) under Manipal Academy of Higher Education (MAHE).
Tissue processing: Primary chondrocytes obtained from normal parts of the articular cartilage were carefully prepared as per the a modified protocol described elsewhere. 3 Details of methodology and the establishment of 2D and 3D cultures are elaborated here below.
Two-dimensional cultures: The intact parts of the respective AC tissue was isolated from the rest of the tissue and processed by mincing in small volumes of DMEM in a 60 mm petri dish. The DMEM was then removed carefully. To the minced tissue, 0.25% tryspin was added. These tissue dishes were placed inside a sterile incubator with a controlled temperature of 37°C for 1 hour. Once the chondrocytes were obtained they were subjected for tryspin treatment, centrifuged and counted. The freshly separated chondrocytes were plated at an average of 1.8 × 104 cells/cm2, in T-25 culture that was seed and maintained in NDM for a period of 21 days by ensuring that the media was changed on alternative days. Eventually, the cells were cryopreserved or processed, as the case may be for cellular molecular analysis and 3D cultures.
Three-dimensional cultures: Well grown chondrocytes on 2D cultures for 21 days (passage 0 [P0]) or [P5] days were eventually suspended in collagen type I gel (made up with 2 mg/ml in alkaline PBS) at a concentration of 3 × 104 cells/ml; 80 μl of this cell suspension was added to Costar 24-well culture inserts placed in a twenty-four cell culture plate, under sterile conditions. The same amount of plain collagen gels without cells was utilized as the control. Both gels remained still and undisturbed for a period of 30 to 40 minutes at 37°C, following, which the gel solidified; during this point, 200 μl of NDM was added to the cell culture plate and 100 μl was added to the inserts consisting of gel along with cells in each well. 3D cultures were maintained for 15 days with twice-weekly media changes. The same experimental process was replicated in 6-well cell culture inserts from costar but 75 × 104 cells/ml were seeded along with 2ml NDM media in each cell culture plate and 1ml in each of the inserts. After this period, the sham control and the experimental groups were exposed to NMcFRB device (sham control with device switched off). Cytochemical staining, immunofluorescence and optical microscopy of the cultures were carried out.
MTT assay: MTT assay is a colorimetric technique for assessing cell metabolic activity. The 2D cell cultures were categorized into two groups: one without exposure to NMcFRB termed as Sham Control (with device turned off but with all other conditions being identical). The experimental group cell culture plate was exposed to NMcFRB, under the exposure protocol. The protocol is described below. The 2D cell culture plate was observed for almost 60% confluency in 48-well cell culture plates. From plates of both the SC and NMcFRB exposed cell cultures, 100 μl of the NDM media was removed carefully and the MTT reagent (5 mg/ml) (which was prepared in 10 ml of PBS) was added to the cell culture plates and placed in a sterile incubator at 37°C for 3 hours undisturbed. Later the cell culture was removed from the incubator and set under an inverted microscope to observe the presence of crystal formation. Once crystals were formed, the MTT reagent along with media was removed and 100 μl of DMSO was added to cell culture plate and kept on a rocker for 5 minutes incubation period. This cell culture was subjected to spectrophotometry to assess cell viability.
Cytochemical staining: The 2D cell culture of P5 — categorized as Sham control, that did not receive any exposure to NMcFRB, and experimental group cultures that were exposed to NMcFRB were both grown in NDM and stained with various stains like Masson trichrome for total collagen, toluidine blue for proteoglycans, hematoxylin and eosin, Safranin-O for fibrillar collagen. Similarly, for the 3D culture, the gel was removed carefully from the 6-24 cell culture insert plates by using a sterile forceps. The inserts were held firmly, and using a sterile blade, the corners of the inserts were cut, the gel was removed with a sterile forceps, and placed in a 35 mm Petri dish and washed with a plain DMEM. The gel was removed from the insert mesh and washed again with PBS. This was now placed on a clean glass slide and fixed with specific fixative and stained with Masson Trichrome, Toluidine blue, Safranin-O, Hematoxylin and Eosin.
Immunofluorescence: For 2D/3D cell cultures of chondrocytes, the cells were initially grown in 48-well cell culture plates (BD Falcon) in a similar man- ner described above. The cell plate categorized as Sham control, without exposure to NMcFRB and the experimental cell culture plate was exposed to NMcFRB. The experimental cells grown in 2D cultures was washed with sterile PBS once, fixed with 4% paraformaldehyde for 20mins, and washed with sterile PBS twice. Blocking non-specific antibodies of the cells were treated with three percent Bovine Serum Albumin for 30mins. The cells were treated with primary antibodies like Collagen type I and Collagen type II, Aggrecan, CD44, and Hsp 70 with a dilution factor of 1:1000 μl respectively overnight at 4°C. Later, cells were washed with PBS thrice and Alexa Flour 594 goat anti-rabbit IgG was added and plates were placed on a rocker for one hour at humid temperature. The cells were rinsed with PBS thrice and Fluoroshield DAPI was added. Plates remained at ambient room temperature for 5 to 10 minutes and observed for a specific antibody using a Fluorescent microscope (Olympus BX51).
Nuclear magnetic coupled fast radio burst set-up: The 2D and 3D chondrocyte cells were exposed to nuclear magnetic coupled fast radio burst (NMcFRB) with laser guided focus around 360° exposure of the target. The exposure protocol was as follows (1) the NMcFRB machine was installed inside a sterile incubator (N-BIOTECH), by a qualified engineer from CARD and connected to the external power source carefully. (2) The machine was switched on and by using a Gauss meter the magnetic field was measured to check if the machine is functional (the NMcFRB machine was precalibrated with a radio spectrum analyzer). (3) The 2D plates were placed on the machine for 1 hour keeping the incubator doors closed. (4) After 1 hour the 2D plate was removed carefully and placed inside another sterile incubator, kept ready for the purpose. (5) With the NMcFRB machine switched off, once again with the Gauss meter the magnetic field was measured (the earth’s magnetic field ambient magnetic field) was measured, to make sure that the machine was switched off. The 2 D plate of Sham control (SC) was placed inside the same incubator where the machine was kept switched off. The same protocol was followed for Sham control of 3D chondrocyte cultures as well. Since the incubator is a sealed metal enclosure, interference of external stray electromagnetic fields would be minimal and further the ambient magnetic field measured would include the magnetic field generated by the electrical and electronic circuits inside the incubator, the ambient magnetic field was between 35 and 45 μT, correlating well with the expected earth’s magnetic field in Bangalore, India (12.9716° N, 77.5946° E). The setup of the machine is shown in Figure 4.
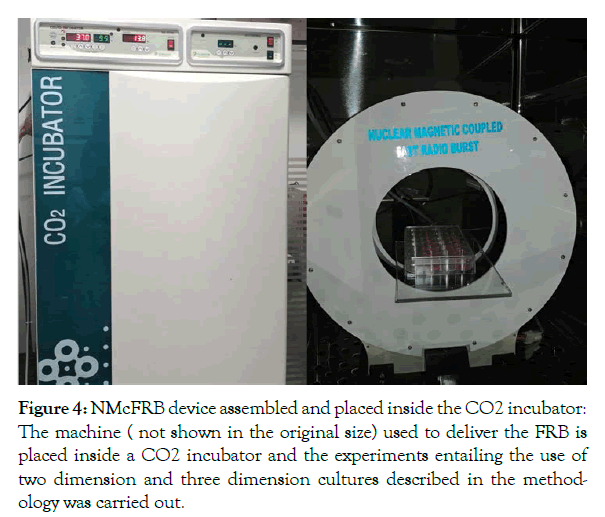
Figure 4: NMcFRB device assembled and placed inside the CO2 incubator: The machine ( not shown in the original size) used to deliver the FRB is placed inside a CO2 incubator and the experiments entailing the use of two dimension and three dimension cultures described in the methodology was carried out.
Exposure nuclear magnetic coupled fast radio burst
The 2D and 3D study chondrocyte culture was placed on the NMcFRB machine, specially designed to fit inside an incubator in which the plates were exposed. Later the same was replaced for sham control (SC) plate of both 2D and 3D chondrocyte cultures were placed, while on keeping the NMcFRB that maintained inside the incubator the machine switched off. While FRB signals are switched on/off and expressed, presence of the ’above the ambient’ magnetic fields was measured with a Gauss meter as shown in Figure 5 for P5.
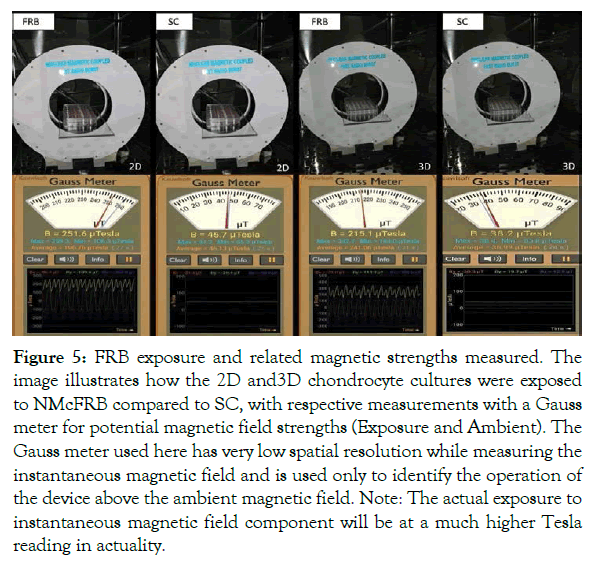
Figure 5: FRB exposure and related magnetic strengths measured. The image illustrates how the 2D and3D chondrocyte cultures were exposed to NMcFRB compared to SC, with respective measurements with a Gauss meter for potential magnetic field strengths (Exposure and Ambient). The Gauss meter used here has very low spatial resolution while measuring the instantaneous magnetic field and is used only to identify the operation of the device above the ambient magnetic field. Note: The actual exposure to instantaneous magnetic field component will be at a much higher Tesla reading in actuality.
MTT assay
MTT ASSAY results for cell viability determination: Considerable increase in cell viability of 2D cultures in Passage 5 exposed for 1 hour with NMcFRB was observed compared to unexposed cultures in the SC, showing very minimal cellular viability which were indicated in Figure 6.
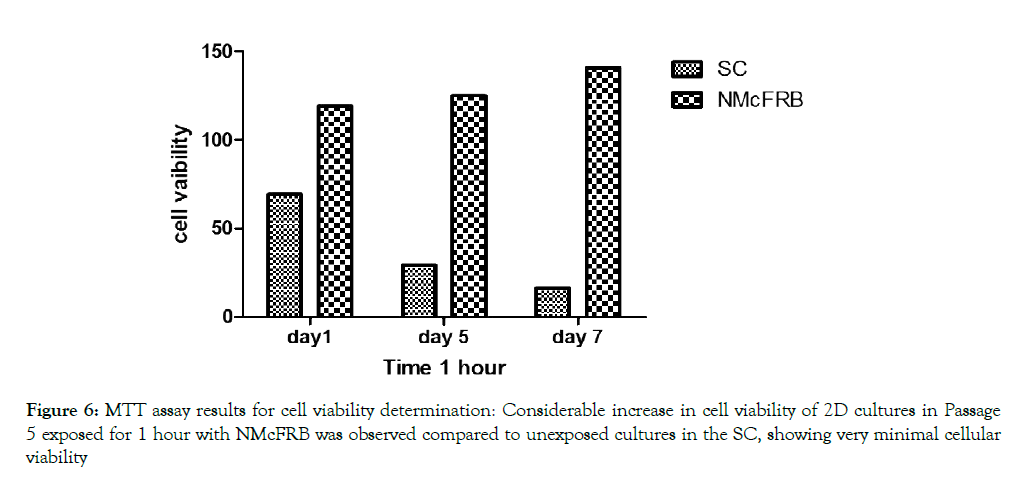
Figure 6: MTT assay results for cell viability determination: Considerable increase in cell viability of 2D cultures in Passage 5 exposed for 1 hour with NMcFRB was observed compared to unexposed cultures in the SC, showing very minimal cellular viability
Culture in two dimensions
Primary chondrocytes obtained from normal L-III of the AC were maintained in NDM for a total period of 21 days (P0) or 10 days (P5). The (P5) cells were categorized into two different conditions: SC and NMcFRB group and grown in NDM media respectively. We clearly noticed that there was increase in cell growth and ECM production in cells exposed to NMcFRB when compared with cells grown in SC without NMcFRB exposure. These images were also captured with a phase contrast microscope in Figure 7 with cytochemical staining. To confirm the finding, we performed immunofluorescence of Col I, Col II, CD 44 and Hsp70 and the results from the representative experiment are shown in Figure 8 (similar observation was seen in 3 different experimental groups). Col II, Agg and Hsp70 in cells exposed to NMcFRB was significantly expressed whereas CD44 and Col I were sustained at lower levels in P5, while the non-expressed SC showed lower levels of Col I, CDD44 and absence of Hsp70. Therefore, our results reproducibly show that P5 chondrocytes grown in NDM on exposure to NMcFRB exhibits matrix properties very similar to that of L-III in the native articular cartilage.
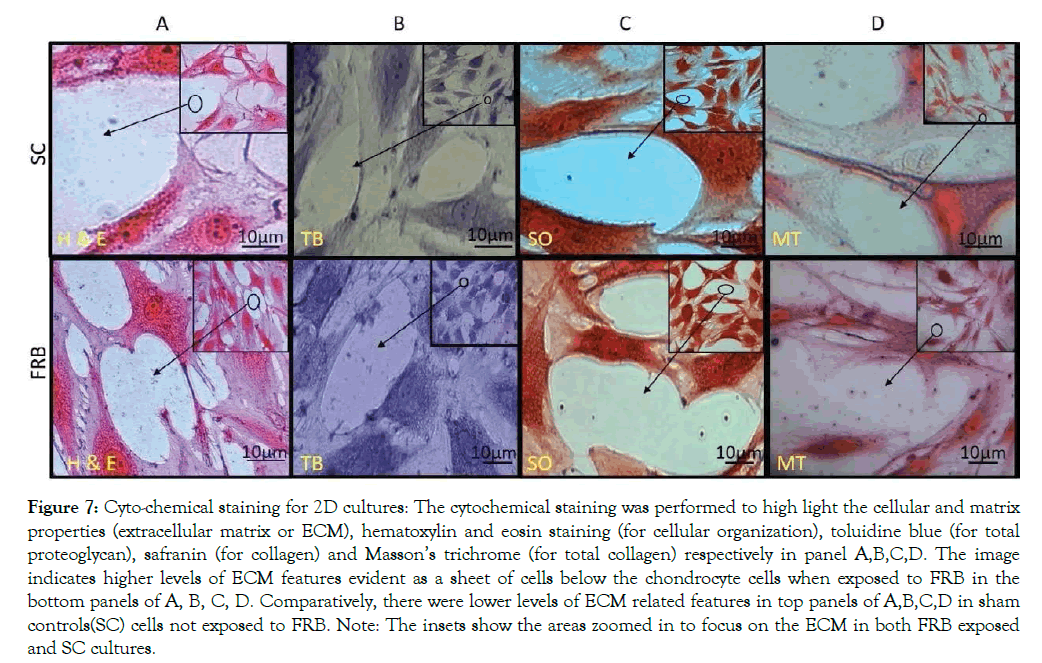
Figure 7: Cyto-chemical staining for 2D cultures: The cytochemical staining was performed to high light the cellular and matrix properties (extracellular matrix or ECM), hematoxylin and eosin staining (for cellular organization), toluidine blue (for total proteoglycan), safranin (for collagen) and Masson’s trichrome (for total collagen) respectively in panel A,B,C,D. The image indicates higher levels of ECM features evident as a sheet of cells below the chondrocyte cells when exposed to FRB in the bottom panels of A, B, C, D. Comparatively, there were lower levels of ECM related features in top panels of A,B,C,D in sham controls(SC) cells not exposed to FRB. Note: The insets show the areas zoomed in to focus on the ECM in both FRB exposed and SC cultures.
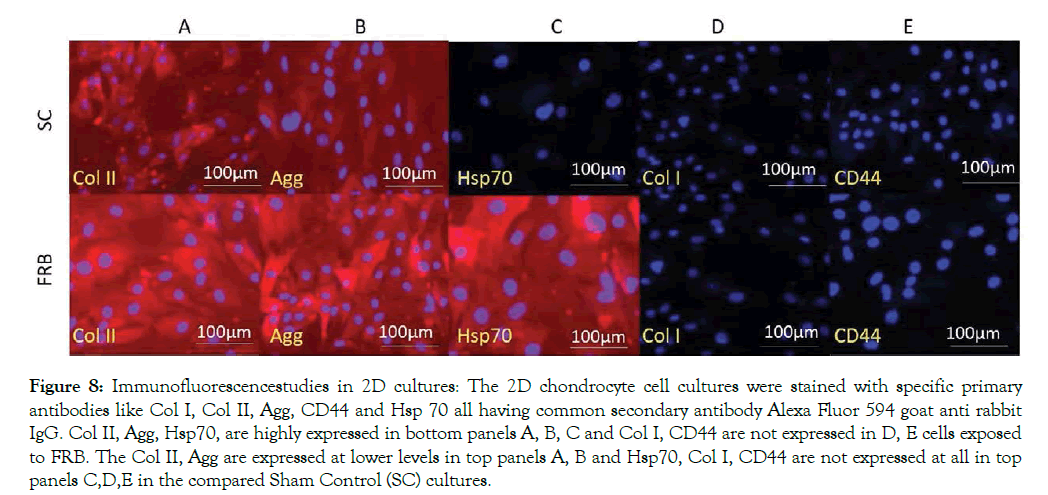
Figure 8: Immunofluorescencestudies in 2D cultures: The 2D chondrocyte cell cultures were stained with specific primary antibodies like Col I, Col II, Agg, CD44 and Hsp 70 all having common secondary antibody Alexa Fluor 594 goat anti rabbit IgG. Col II, Agg, Hsp70, are highly expressed in bottom panels A, B, C and Col I, CD44 are not expressed in D, E cells exposed to FRB. The Col II, Agg are expressed at lower levels in top panels A, B and Hsp70, Col I, CD44 are not expressed at all in top panels C,D,E in the compared Sham Control (SC) cultures.
Culture in three dimensions
Primary Chondrocytes multiplied in cultures of 2D were placed in collagen type I gel in Costar culture inserts; the gels were incubated without cells that were maintained for 15 days in two group, SC and NMcFRB group grown in NDM observed in Figure 8 after which were processed for observation with an inverted microscope to examine their growth. Immunofluorescence technique of deep layer III, and cytochemical characterized method showed the presences of ECM. Cells were grown on the surface of the gel that was visualized in SC (A) and NMcFRB (B) which are indicated in Figure 9. The surface of the gels that were exposed to NMcFRB showed thick, dense collagen fibers and round chondrocyte cell formation in the deep layer III, where as thin, lower levels of collagen fibers and very few round chondrocyte cells formed in SC. This indicated that NMcFRB exposed group shows significantly more presence of the chondrocytes when compared to SC. This alone can modify the gel surface organization for L-III (Figure 10). The gels with the chondrocytes were stained with H&E, TB, MT and S-O as seen in Figure 11. IMcFRB exposure multiplied HC tissue distinctly showed an organized multilayered (four layer) structure with sparse cell number and significantly increased levels of total PG and collagen in L-III. Gels that were grown in SC had lower levels of total PG and collagen when compared to NMcFRB exposed group. Immunofluorescence technique for Col I, Agg, CD44 and Col II of 3D regenerated HC tissue is observed in Figure 12. NMcFRB regenerated HC distinctly showed the 4 discrete layers of AC, when appropriately enhanced L-III properties for Col II, Agg and Hsp70. Features associated with fibrous and elastic cartilage as in Col I and CD44 were either decreased or absent. Interestingly the 3D gel grown in SC also showed higher level of Col II, Agg ’but absence of Hsp70’, and lower levels of the Col I and CD44 when compared to NMcFRB group under exactly the same condition of growth in NDM. This indicates that by using NDM, 3D gels can be modified with the help of exposure of NDM 3D gel to NMcFRB mimicking the human articular cartilage in HC. NMcFRB seems to play a role in reprogramming these cells show in Figure 13.
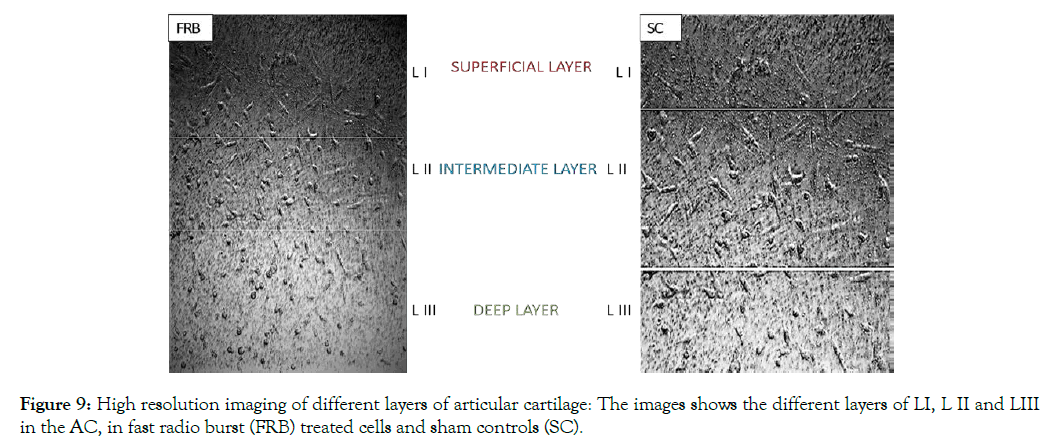
Figure 9: High resolution imaging of different layers of articular cartilage: The images shows the different layers of LI, L II and LIII in the AC, in fast radio burst (FRB) treated cells and sham controls (SC).

Figure 10: High power resolution of deep layer III: Figure specifically shows details in the deep layer L III. When the surface of the gel is exposed to FRB it showed thick, dense collagen fibres, round chondrocytes cell formation without lacunae (black arrows) in the deep layer III. Comparatively, there were thin, low levels of collagen fibres and very few round chondrocyte cells (black arrows) formed in SC. Natural collagen gel is indicated by red arrows. These changes were observed gradually on days 5x, 10x and 15x, magnified at 20x.
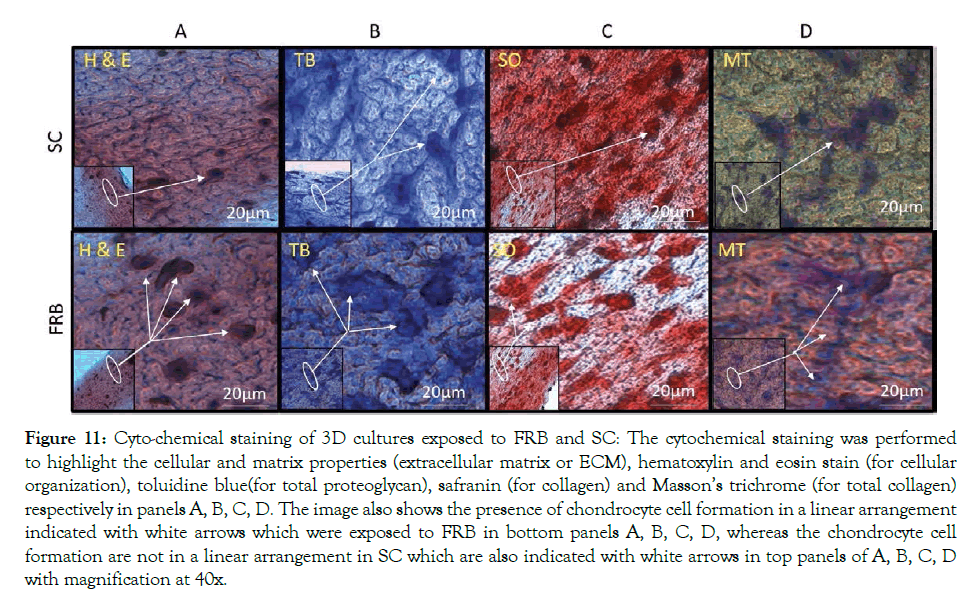
Figure 11: Cyto-chemical staining of 3D cultures exposed to FRB and SC: The cytochemical staining was performed to highlight the cellular and matrix properties (extracellular matrix or ECM), hematoxylin and eosin stain (for cellular organization), toluidine blue(for total proteoglycan), safranin (for collagen) and Masson’s trichrome (for total collagen) respectively in panels A, B, C, D. The image also shows the presence of chondrocyte cell formation in a linear arrangement indicated with white arrows which were exposed to FRB in bottom panels A, B, C, D, whereas the chondrocyte cell formation are not in a linear arrangement in SC which are also indicated with white arrows in top panels of A, B, C, D with magnification at 40x.
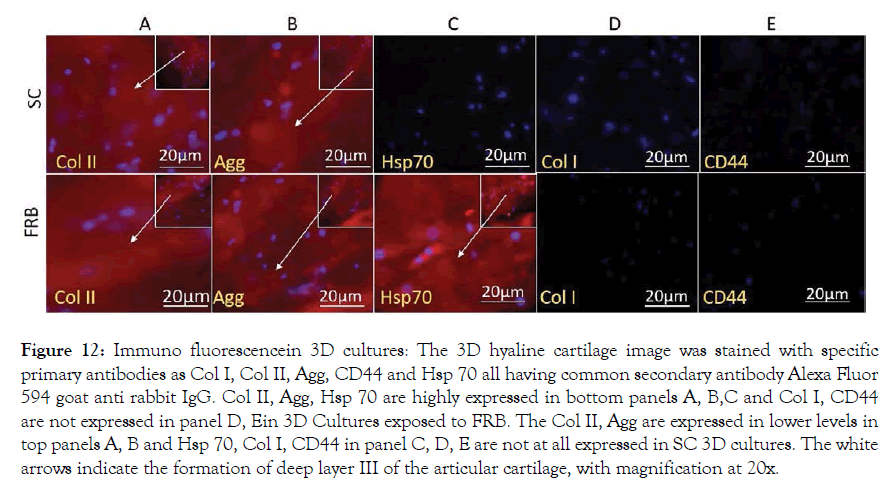
Figure 12: Immuno fluorescencein 3D cultures: The 3D hyaline cartilage image was stained with specific primary antibodies as Col I, Col II, Agg, CD44 and Hsp 70 all having common secondary antibody Alexa Fluor 594 goat anti rabbit IgG. Col II, Agg, Hsp 70 are highly expressed in bottom panels A, B,C and Col I, CD44 are not expressed in panel D, Ein 3D Cultures exposed to FRB. The Col II, Agg are expressed in lower levels in top panels A, B and Hsp 70, Col I, CD44 in panel C, D, E are not at all expressed in SC 3D cultures. The white arrows indicate the formation of deep layer III of the articular cartilage, with magnification at 20x.
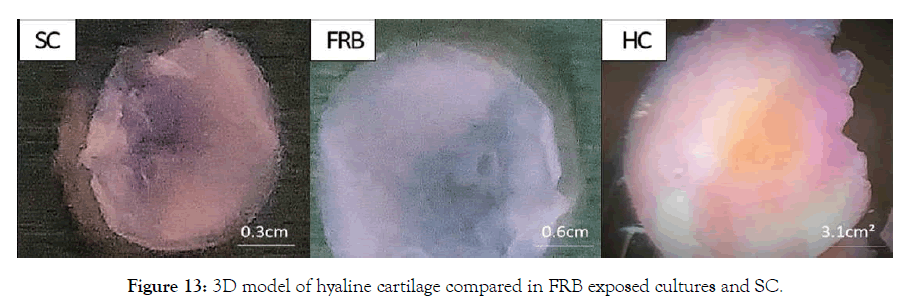
Figure 13: 3D model of hyaline cartilage compared in FRB exposed cultures and SC.
Exposure nuclear magnetic coupled fast radio burst
The 2D and 3D study chondrocyte culture was placed on the NMcFRB machine, specially designed to fit inside an incubator in which the plates were exposed. Later the same was replaced for sham control (SC) plate of both 2D and 3D chondrocyte cultures were placed, while on keeping the NMcFRB that maintained inside the incubator the machine switched off. While FRB signals are switched on/off and expressed, presence of the ’above the ambient’ magnetic fields was measured with a Gauss meter as shown in Figure 5 for P5.

Figure 5: FRB exposure and related magnetic strengths measured. The image illustrates how the 2D and3D chondrocyte cultures were exposed to NMcFRB compared to SC, with respective measurements with a Gauss meter for potential magnetic field strengths (Exposure and Ambient). The Gauss meter used here has very low spatial resolution while measuring the instantaneous magnetic field and is used only to identify the operation of the device above the ambient magnetic field. Note: The actual exposure to instantaneous magnetic field component will be at a much higher Tesla reading in actuality.
MTT assay
MTT ASSAY results for cell viability determination: Considerable increase in cell viability of 2D cultures in Passage 5 exposed for 1 hour with NMcFRB was observed compared to unexposed cultures in the SC, showing very minimal cellular viability which were indicated in Figure 6.

Figure 6: MTT assay results for cell viability determination: Considerable increase in cell viability of 2D cultures in Passage 5 exposed for 1 hour with NMcFRB was observed compared to unexposed cultures in the SC, showing very minimal cellular viability
Culture in two dimensions
Primary chondrocytes obtained from normal L-III of the AC were maintained in NDM for a total period of 21 days (P0) or 10 days (P5). The (P5) cells were categorized into two different conditions: SC and NMcFRB group and grown in NDM media respectively. We clearly noticed that there was increase in cell growth and ECM production in cells exposed to NMcFRB when compared with cells grown in SC without NMcFRB exposure. These images were also captured with a phase contrast microscope in Figure 7 with cytochemical staining. To confirm the finding, we performed immunofluorescence of Col I, Col II, CD 44 and Hsp70 and the results from the representative experiment are shown in Figure 8 (similar observation was seen in 3 different experimental groups). Col II, Agg and Hsp70 in cells exposed to NMcFRB was significantly expressed whereas CD44 and Col I were sustained at lower levels in P5, while the non-expressed SC showed lower levels of Col I, CDD44 and absence of Hsp70. Therefore, our results reproducibly show that P5 chondrocytes grown in NDM on exposure to NMcFRB exhibits matrix properties very similar to that of L-III in the native articular cartilage.

Figure 7: Cyto-chemical staining for 2D cultures: The cytochemical staining was performed to high light the cellular and matrix properties (extracellular matrix or ECM), hematoxylin and eosin staining (for cellular organization), toluidine blue (for total proteoglycan), safranin (for collagen) and Masson’s trichrome (for total collagen) respectively in panel A,B,C,D. The image indicates higher levels of ECM features evident as a sheet of cells below the chondrocyte cells when exposed to FRB in the bottom panels of A, B, C, D. Comparatively, there were lower levels of ECM related features in top panels of A,B,C,D in sham controls(SC) cells not exposed to FRB. Note: The insets show the areas zoomed in to focus on the ECM in both FRB exposed and SC cultures.

Figure 8: Immunofluorescencestudies in 2D cultures: The 2D chondrocyte cell cultures were stained with specific primary antibodies like Col I, Col II, Agg, CD44 and Hsp 70 all having common secondary antibody Alexa Fluor 594 goat anti rabbit IgG. Col II, Agg, Hsp70, are highly expressed in bottom panels A, B, C and Col I, CD44 are not expressed in D, E cells exposed to FRB. The Col II, Agg are expressed at lower levels in top panels A, B and Hsp70, Col I, CD44 are not expressed at all in top panels C,D,E in the compared Sham Control (SC) cultures.
Culture in three dimensions
Primary Chondrocytes multiplied in cultures of 2D were placed in collagen type I gel in Costar culture inserts; the gels were incubated without cells that were maintained for 15 days in two group, SC and NMcFRB group grown in NDM observed in Figure 8 after which were processed for observation with an inverted microscope to examine their growth. Immunofluorescence technique of deep layer III, and cytochemical characterized method showed the presences of ECM. Cells were grown on the surface of the gel that was visualized in SC (A) and NMcFRB (B) which are indicated in Figure 9. The surface of the gels that were exposed to NMcFRB showed thick, dense collagen fibers and round chondrocyte cell formation in the deep layer III, where as thin, lower levels of collagen fibers and very few round chondrocyte cells formed in SC. This indicated that NMcFRB exposed group shows significantly more presence of the chondrocytes when compared to SC. This alone can modify the gel surface organization for L-III (Figure 10). The gels with the chondrocytes were stained with H&E, TB, MT and S-O as seen in Figure 11. IMcFRB exposure multiplied HC tissue distinctly showed an organized multilayered (four layer) structure with sparse cell number and significantly increased levels of total PG and collagen in L-III. Gels that were grown in SC had lower levels of total PG and collagen when compared to NMcFRB exposed group. Immunofluorescence technique for Col I, Agg, CD44 and Col II of 3D regenerated HC tissue is observed in Figure 12. NMcFRB regenerated HC distinctly showed the 4 discrete layers of AC, when appropriately enhanced L-III properties for Col II, Agg and Hsp70. Features associated with fibrous and elastic cartilage as in Col I and CD44 were either decreased or absent. Interestingly the 3D gel grown in SC also showed higher level of Col II, Agg ’but absence of Hsp70’, and lower levels of the Col I and CD44 when compared to NMcFRB group under exactly the same condition of growth in NDM. This indicates that by using NDM, 3D gels can be modified with the help of exposure of NDM 3D gel to NMcFRB mimicking the human articular cartilage in HC. NMcFRB seems to play a role in reprogramming these cells show in Figure 13.

Figure 9: High resolution imaging of different layers of articular cartilage: The images shows the different layers of LI, L II and LIII in the AC, in fast radio burst (FRB) treated cells and sham controls (SC).

Figure 10: High power resolution of deep layer III: Figure specifically shows details in the deep layer L III. When the surface of the gel is exposed to FRB it showed thick, dense collagen fibres, round chondrocytes cell formation without lacunae (black arrows) in the deep layer III. Comparatively, there were thin, low levels of collagen fibres and very few round chondrocyte cells (black arrows) formed in SC. Natural collagen gel is indicated by red arrows. These changes were observed gradually on days 5x, 10x and 15x, magnified at 20x.

Figure 11: Cyto-chemical staining of 3D cultures exposed to FRB and SC: The cytochemical staining was performed to highlight the cellular and matrix properties (extracellular matrix or ECM), hematoxylin and eosin stain (for cellular organization), toluidine blue(for total proteoglycan), safranin (for collagen) and Masson’s trichrome (for total collagen) respectively in panels A, B, C, D. The image also shows the presence of chondrocyte cell formation in a linear arrangement indicated with white arrows which were exposed to FRB in bottom panels A, B, C, D, whereas the chondrocyte cell formation are not in a linear arrangement in SC which are also indicated with white arrows in top panels of A, B, C, D with magnification at 40x.

Figure 12: Immuno fluorescencein 3D cultures: The 3D hyaline cartilage image was stained with specific primary antibodies as Col I, Col II, Agg, CD44 and Hsp 70 all having common secondary antibody Alexa Fluor 594 goat anti rabbit IgG. Col II, Agg, Hsp 70 are highly expressed in bottom panels A, B,C and Col I, CD44 are not expressed in panel D, Ein 3D Cultures exposed to FRB. The Col II, Agg are expressed in lower levels in top panels A, B and Hsp 70, Col I, CD44 in panel C, D, E are not at all expressed in SC 3D cultures. The white arrows indicate the formation of deep layer III of the articular cartilage, with magnification at 20x.

Figure 13: 3D model of hyaline cartilage compared in FRB exposed cultures and SC.
We have here described a new modality to regenerate multilayered AC tissue in three-dimensional cultures of primary chondrocytes developed from native AC. Our methodology showed significant evidence those properties of native AC could be maintained in our culture system more efficiently than by methods reported earlier. The significant upsides of our methodology are discussed. Primary two-dimensional cultures of AC chondrocytes obtained from non-human sources have been reported; however, literature on the culture of normal human autologous articular cartilage exposed to NMcFRB is very few in number. Most of these reports on human chondrocytes have not elaborated the quality and quantity of extracellular matrix secreted by cultured cells, as they have focused mainly on the gene expression profile. In our method, human chondrocytes multiplied in UCS containing media in two different groups- Sham controls and with NMcFRB exposure groups are detailed. NMcFRB exposed 2D cells showed substantial matrix formation, which was rich in collagen II and aggrecan with the absence or expression of CD44 and collagen I when compared to unexposed SC. This is the first demonstration of the sustenance of AC like ECM properties in 2D cultures of human chondrocytes properties with NMcFRB exposure together with the upregulation of the Hsp70. As the native AC comprises more than 70 percent of matrix components, it is important that extracellular matrix properties of cultured chondrocytes has to be as identical as that of native AC. Interestingly, in NMcFRB grown chondrocytes, we observed the upregulation of Hsp 70 and ECM formation close to native AC. Immunofluorescence of cells was better in P5 than in P1 cells. Earlier studies on primary chondrocytes culture had shown reduced AC specific markers after the first passage.
P5 chondrocytes multiplied in 2D cultures were placed in collagen I gels and further cultured in two different sets: SC and NMcFRB exposure groups containing the same growth media NDM as the 3D module. We observered that cells growing in three dimensions altered the collagen gel properties very significantly after 15 days and produced tissue of approximately 2 mm thickness. This generally is the observed thickness of native AC in the human knee joint, which shares many features and properties of native HC, as four layered organization that have different levels of extra cellular matrix specific protein expression, of varying cell density is at different layers, a middle layer with same properties as seen in LIII of native HC. Analysis of this LIII like region in the regenerated new tissues showed that it contained higher levels of Col II, Agg and Hsp70 and lower levels of Col I and absence of CD44. Thus, the regenerated/reconstructed tissue portrayed most features of the native HC except that the L-III that consists of cells that aren’t encircled by lacunae.
Presently available methods fail to replicate HC like tissue properties; this has shown degradation of the implanted tissue/ cells shortly upon implantation. The HC tissue regenerated in our 3D cultures showed multilayered cellular and ECM organization which is very similar to the native articular cartilage tissue. However, we have not performed any specific compressive and mechanical strength measurements of the tissue, we propose that, as the regenerated tissue is identical to native AC, the tissues grown in our cultures could be more durable when used in vivo. Though not clinically evaluated, this regenerated tissue could be used as a single step protocol as autologous cartilage implants. The primary chondrocyte derived from two-dimensional cultures grown in newly defined medium and exposed to NMcFRB may be cryopreserved and used for growing three-dimensional cultures as and when required.
We have reported a novel methodology to regenerate and reconstruct articular cartilage tissue from primary chondrocytes cultured in NDM and not xenogeneic media and exposing them to Nuclear Magnetic coupled Fast Radio Burst — A new science that leads to upregulation of Hsp70. As our method is reproducible and yields articular cartilage with all significant features of the HC, we propose that, after further optimization of this process, the regenerated autologous articular cartilage can be clinically implanted to replace grade L-III or IV degraded AC using this cost effective procedure.
We express our deepest gratitude to the staff and management of the Manipal Institute of Regenerative Medicine, (MAHE) and Organization de Scalene for contribution of their valuable time, guidance and financial support for this study. Special thanks to the team at the Centre for Advanced Research and Development for providing us the device to produce the “Nuclear Magnetic Coupled fast Radio Burst” demonstrated promising results for the upregulation of Heat Shock Proteins in human Chondrogenesis. We also thank the staff and management of Raja Rajeswari Medical College and Hospital for providing the tissue samples and the umbilical cord blood, for the duration of the project.
Relevant data are obtained upon request.
There was no special funding for this work.
No competing interest declared.
Citation: Pillai VK et.al, (2019) Nuclear Magnetic Coupled Fast Radio Burst Induced Upregulation of Hsp70 in Regeneration of Human Chondrocytes- A Sham Control Study. J Cell Sci Ther. doi: 10.35248/2157-7013.19.10.292
Received: 17-Jul-2019 Accepted: 01-Oct-2019 Published: 09-Oct-2019 , DOI: 10.35248/2157-7013.19.10.292
Copyright: © 2019 Pillai VK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.