Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Review Article - (2025)Volume 16, Issue 3
Nowadays, MXene is considered the newest family of layered 2D materials, thus attracting great attention of researchers all over the world. As its greatest advantages, the outstanding mechanical strength, extraordinary hydrophilicity, distinct surface chemistry as well as possibility to delamination on monolayer nanoplatelets with a high aspect ratio can be mentioned. Due to the growing numbers of research reports on MXene-based materials, this review has been designed to systematize the knowledge of the synthesis methods and the wide application possibilities of these 2D materials. Therefore, the focus will be put on the synthesis and characterization of MXene containing single and multi-transition metals. Moreover, the attention will be extended to study of the applications of MXene that can be employed as supercapacitors, catalysts and photocatalysts, biomaterials and many others. The approach of this review presents the current state-of-the-art which is required to explore new research directions.
MXene; 2D materials; Layered structure; Photocatalysts; Biomaterials; Nanomaterials
MXene is the newest family of layered 2D materials that attracts great attention of researchers all over the world. The interest of MXene which results in a rapidly growing number of research reports is due to their numerous and fascinating features with extraordinary technological importance. The first notification of MXene was reported in 2011 by Gogotsi, et al., that was obtained by etching the Al layer from Ti3AlC2 MAX phase. Basically, MAX phases are referred with the formula Mn+1AXn, from which the “A” layer can be etched to produce “MXene”. Usually, “A” belongs to group 13 or 14 elements while “M” is early transition metal (Sc, Ti, V, Cr, Zr, Nb, Mo, Hf and Ta) element and “X” can be C or N. The resulting general formula for the MXene is Mn+1XnTx, where “T” stands for OH-, X- or any other functional groups [1]. Beyond 2011, the investigation of MXenes has led to the discovery of new structures based on the chemical composition. The reasons of the high interest for MXene are the strong hydrophilic nature and the promising electronic conductivities. The unique combination of these properties along with easy synthesis of MXene makes them promising materials for different applications. As per literature reports, MXene have been found in various technologies including electrochemical energy storage, supercapacitors, biosensors, transparent conductive electrodes and water purifier. As per SCOPUS database, there are a total of 8450 articles published on MXene and MXene-based materials since 2011 (MXene and MXene-based materials were used as keywords) (Figure 1 (left side)). Majority of the articles are focused on the synthesis of new compositions of MXene and their utilization as energy storage materials, biosensors, bioimaging and photocatalyst. Regarding the current environmental issues related to its remediation and the production of green energy, only 230 articles (2% of total existing literature) involving MXene in H2 production and 58 articles (1% of total existing literature) on wastewater treatment are reported (Figure 1 (right side)). Therefore, the approach of environmental issues using MXene appears very open and not yet comprehensively explored [2].
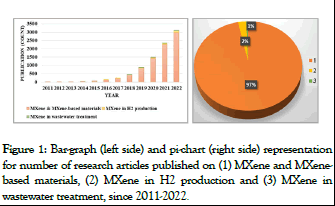
Figure 1: Bar-graph (left side) and pi-chart (right side) representation for number of research articles published on (1) MXene and MXenebased materials, (2) MXene in H2 production and (3) MXene in wastewater treatment, since 2011-2022.
Presently, exploration of MXene based on processing, engineering and performance, is a hot area of research (Figure 2). The ongoing research is focusing on the following aspects of MXene: (i) Utilization of existing and newly synthesized MAX and non-MAX phases as precursor material for easy, scalable, reproducible and cost-effective synthesis of MXene, (ii) Design and development of new compositions of fluoride-free MXene with more than one transition metals, (iii) Stabilization of mono and multi-layer MXene to get suitable optical, magnetic, catalytic and electrical properties. Although there is intense research on MXene, a multitude of structures are waiting to be discovered. Therefore, the need to summarize the current knowledge of synthesis strategies as well as applications of the MXene based materials is substantial. This review summarizes the recent progress on MXene research both in synthesis and application, thus aiming to approach new research directions. Future perspectives for the development of this research area are described at the end of review.
Figure 2: Schematics representation of 2D structured MXene processing, engineering and performance.
Structure of MAX and MXene
Currently, approx 70 MAX phases are reported and majority of these phases existed as layered structures with hexagonal symmetry (space group P63/mmc). These MAX phases may act as precursor materials for MXene fabrication. Generally, in MAX structure “M” atoms form a closed packing arrangement with “X” atoms occupying the octahedral sites. The Mn+1Xn layers are interleaved by the “A” layers (Figure 3). The crystal structures of MAX phases can be classified according to their composition (Figure 3). Comparing to other layered materials where there are weak van der Waals forces to hold the structure together (like graphite), the MAX phases demonstrate a structure with the presence of mixed covalent-metallic-ionic character in the strong M-X bond and completely metallic character in M-A bond. Due to this, the breakage of bonds by any mechanical forces is challenging. In addition, the difference in strength of M-X and M-A bonds is responsible for the selective etching of ‘A’ layer realized by chemical treatment during the production of MXene [3].
Figure 3: Crystal structure of MAX phases with compositions (a) M2AX, (b) M3AX2, (c) M4AX3.
In accordance with the literature, selective etching of Al layer from Ti3AlC2 by HF treatment conducted at room temperature has been reported for the first time in 2011 by Yury Gogotsi and Michael Barsoum, materials science professors at Drexel University. On immersing Ti3AlC2 in HF, there is a set of possible reactions which are as follows:
Ti3AlC2+3HF → AlF3+3/2 H2+Ti3C2
Ti3C2+2H2O → Ti3C2(OH)2+H2
Ti3C2+2HF → Ti3C2F2+H2
In this study, the stabilization of Ti3C2(OH)2 MXene phase is confirmed using Powder X-Ray Diffraction (PXRD) and Raman analysis. XPS studies performed before and after HF treatment also confirms the stabilization of Ti3C2(OH)2 due to the presence of Ti−C and Ti−O bonds. SEM images reflects layered morphology with basal planes fan out and spread apart due to HF etching (Figure 4).
Figure 4: (a) PXRD pattern of Ti3AlC2, HF-treated product of Ti3AlC2 and exfoliated product after HF treatment along with line diagram of Ti3C2F2 and Ti3C2(OH)2 for comparison purpose. (b) SEM image and (c) XPS spectra of the product obtained after HF treatment and Ti3AlC2.
Other MXene phases also exist in different composition and stoichiometries such as M2XTx, M3X2Tx and M4X3Tx (Figure 5), since there is a variety of MAX phases. Generally, the thickness of one MXene layer is less than one nanometer, whereas their lateral dimensions are of up to ten microns. This means that MXene are the ideal candidate for new type of 2D materials.
Figure 5: Crystal structures of MXene phases with compositions (a) M2XTx, (b) M3X2Tx, (c) M4X3Tx.
Synthesis of MXene
There are almost 40 known MXene compositions which are synthesized by bottom-up and top-down approaches. The bottom-up involves either Chemical Vapor Deposition (CVD) method, template assisted method or Plasma Enhanced Pulsed Laser Deposition (PE-PLD) method. Generally, these methods are utilized to synthesize multilayer 2D transition metal carbides and nitrides with novel and unique stoichiometries for example WC, TaC, Mo2C, Ti3C2Tx etc. However, to fabricate single layer MXene, the top-down approach is employed. This approach involves etching of a layered materials followed by delamination. The etching treatment is performed using HF, electrochemical methods, etc. and some examples of MXene synthesis are compiled in Table 1.
| Compositions | Etching method | Reaction conditions |
| TiC2Tx | HF etching | HF etching, followed by delamination in DMSO |
| Ti3CNTx | Salt+acid etching | HCl+LiF etching to get multilayered Ti3CN, followed by ultrasonication in distilled water to get monolayer |
| TiNbCTx | HF etching | HF etching for 28 hours |
| Ti3C2Tx | Mixed acid etching | HCl+HF etching, followed by delamination using probe ultrasonication to get monolayer flakes |
| Nb2CTx | HF etching | HF etching to get multilayer Nb2CTx, followed by delamination in alkylamine and tetraalkylammonium salt |
| Zr3C2Tx | HF etching | HF etching with observation of bubbling corresponds to H2 and CH4 removal |
| Ti3C2(OH)2 | NaOH assisted Hydrothermal etching | NaOH at 270°C |
| Ti3C2Tx (T=O,OH) |
Electrochemical corrosion | Electrochemical etching using two electrode system, wherein MAX phase is used as cathode and anode. The anode was kept under observation on varying electrolytes i.e. H2SO4, HNO3, FeCl3, NaOH, NH4Cl to get Ti3C2Tx MXene |
| Ti2CTx Cr2CTx V2CTx |
Thermal-assisted electrochemical etching | Etching by applying voltage to get monolayer (optimization of etching time, temperature and electrodes) |
| Ti3C2Cl2 | Water free etching or molten salt etching | Ti3AlC2+ZnCl2 in 1:15 and 1:6 molar ratio, followed by calcination at 550°C for 0.5-5.0 hours to get Ti3ZnC2 and Ti3C2Cl2 |
| Ti3C2Cl2 Ti2CCl2 Nb2CCl2 |
Water free etching or molten salt etching | Different MAX phases such as Ti2AlC, Ti3AlC2, Ti3AlCN, Nb2AlC, Ta2AlC, Ti2ZnC, Ti3ZnC2 treated with CdCl2, FeCl2, CoCl2, CuCl2, AgCl, NiCl2 |
Table 1: Lists of different compositions of MXene obtained by top-down approach.
After the etching of Al layer, the MXene is dispersed in polar organic solvent to form a colloidal solution, thus facilitating its stabilization in thin films, composites or other items. Usually, the polar organic solvents used are DMSO, hydrazine, urea and isopropylamineto swell the MXene layered structure. Since MXene carry negative charge, inorganic metal cations (Li+, Na+, K+, Mg2+, Al3+, Sn4+, Ca2+) are often used for intercalation. In general, the synthesis of single layer MXene can be classified according to the type of the used precursors, i.e., MAX and non- MAX phases (ScAl3C3, Mo2Ga2C, Zr3Al3C5).
Synthesis of MXene from MAX phases
Prior to MXene preparation, MAX phases need to be synthesized. In the pioneering effort of Pietzka and Schuster, Ti3AlC2 MAX phase was prepared by sintering of cold compact powder of TiAl, Al4C3 and carbon in pure hydrogen. Ever since the pioneering work, several methods have been reported for the synthesis of MAX phases, e.g., hot isostatic pressing, selfpropagation high temperature method, spark plasma sintering, mechanochemical, direct carbothermal reduction, electrochemistry method and CVD method. Among the above listed methods, direct carbothermal reduction was the first industrialized method to prepare TiC from titanium and carbon powders at 1773-1973 K. Due to high cost of titanium powder as raw material, carbothermal reduction of TiO2 and carbon powder was considered as commercialized technique. However, this synthetic method has slow solid-solid reaction between transition metal oxides and carbon, thus requiring longer time at higher temperature resulting in the production of uncontrollable free carbon in the form of CO and CO2. To overcome this issue, Zhang, et al. synthesized titanium carbide, titanium nitride and titanium carbonitride by two steps carbothermal reduction process. The first step was focused on the preparation of TiCnO1-n (as precursor) and in second step, the precursor and carbon powder were subjected to ball milling followed by calcination [4]. To get TiC, TiN or TiCxN1-x compositions, calcination was performed under vacuum and/or nitrogen atmosphere. The advantage of this two-step carbothermal method over the other reported methods are the following: (i) The product has high purity and controlled C:N ratio; (ii) Efficient carbon reduction in first step; (iii) The second step helps the stabilization of monophasic product; (iv) The reactant parameters (particle size and stochiometric ratio) have a significant effect on the structure and properties of products. Recently another study from Zhang et al. reported the preparation of Ti3AlC2 powder by microwaveassisted molten salt method. In this study, a very high purity Ti3AlC2 powder was prepared by mixing TiH2, Al and TiC powder in molar ratio 1:1:1.8 in a molten salt of NaCl-KCl under microwave conditions at 1050°C for 30 minutes. Comparing to traditional heat treatment, this method has very mild conditions. In agreement with literature, distinct compositions of MXene have been derived from MAX phases by etching treatment and followed by delamination to get single layer of MXene. Different acids can be used as an etching agent, including HF, HCl, HF +HCl and HCl+LiF. Since etching with different acids improves the stability, flake sizes and stabilizes MXenes with varying chemical compositions. Recently, Liu et al. has summarized the role of different compositions and structures of MXene (Ti3C2Ox, Ti3C2(OH)x, Mo2CTx, Ti3C2Tx nanofibers, Ti3C2Tx flakes, MoSe2/Ti3C2Tx, etc.) that were prepared by different synthetic methods. In this study, the performance of Hydrogen Evolution Reaction (HER) has been considered to assess the experimental parameters and the results of this comparative study are provided in Table 2.
| Synthetic method | Disadvantage | Advantage |
| Fluoride etching | High toxicity of HF Lower HER activity |
Accordion-like structure Small flake size |
| Alkali etching | Formation of some oxide/hydroxides may further hinder the required Al extraction | Avoid the use of HF Remove the amphoteric or acidic atoms from original MAX phase |
| Electrochemical etching | Low yield | Avoid the use of HF Extend range of HF techniques and potential compositions of MXene Fabricate MXene difficult to prepare |
| Water-free etching | High temperature Low hydophilicity |
Expand the selection range of MXene precursor and MAX phase Provide a green and viable route to prepare MXenes |
Table 2: Advantage and disadvantage of MXene synthesized using different methods.
Likely to nanomaterials, MXene also have the possibility to exhibit different dimensionalities depending on synthetic methods and post treatment methods. Thereby based on movement of electrons MXene can be classified as 0D, 1D, 2D and 3D materials. Reported studies have shown that MXenes can be stabilized as nanorod, nanoribbon, quantum dot, porous foam and nanofibers based on synthetic methods. Thereby to get more clarity in the field of MXene synthesis, MXene with 0D, 1D, 2D and 3D structures are summarized. Briefly, the synthesis of different compositions of MXene involves ultrasonic method, ball milling, electrospinning, refluxing method, hydrothermal solvothermal and many more. It also provides the application outputs of MXene depending on their shape, size and dimensions. A detailed description of applications of MXene is discussed in upcoming section 4 [5].
Synthesis of MXene from non-MAX phases
MXene can be prepared by employing non-MAX phases as precursor materials. For instance, layered carbides with the formula (MC)n[Al(A)]mCm-1 are well known non-MAX phase that can be utilized to prepare MXene. In such a precursor, “M” is an early transition metal and “A” corresponds to Si or Ge. There are more than 30 members which have been reported as non-MAX phases like YAl3C3, CeAl3C3, DyAl3C3, ErAl3C3, TmAl3C3, Zr3Al3C5, ZrAl4C4, ZrAl8C7, Zr2Al4C5, Zr3Al4C6, Zr2[Al(Si)]4C5, Zr3[Al(Si)]4C6, [(ZrY)]2Al4C5, Zr2[Al(Ge)]4C5, Zr3[Al(Ge)]4C6, Zr[Al(Si)]8C7, Zr[Al(Si)]4C4, Hf2Al4C5, Hf3Al4C6, HfAl4C4, etc. Among these non-MAX phases, Zr3Al3C5 is the first material that has been employed as non- MAX phase to prepare MXene swhere the etching treatment of Zr3Al3C5 leads to the etching of Al3C3 layer instead of pure Al layer. Hf3C2Tx is another example of MXene that is obtained by utilizing Hf3(Al, Si)4C6 non-MAX phase. In this case, the presence of Si is to facilitate the etching process by weakening of the existing adhesiveness between Hf-C and Al(Si)-C layers. Moreover, Halim et al. has synthesized Mo2CTx MXene from a non-MAX phase that does not contain Al. Indeed, by etching of Ga from Mo2Ga2C to prepare Mo2CTx, a new door to synthesize MXene has been opened. In addition, very recently, Sanal et al. has reported the synthesis of Mo2CTx by a fluoride free etching process. By using a non-MAX phase precursor based on Mo-In-C, UV-assisted etching in presence of phosphoric acid has been successfully employed to prepare Mo2CTx.
Double transition metal MXene
Gogotsi and Barsoum, et al. has shown that not only homometallic MXene but heterometallic MXene also exist. They have used Density Functional Theory (DFT) to predict the stability of more than 20 new compositions of ordered doubletransition metal MXene with formula M2'M''C2 and M2'M2''C3. Here, M' (outer layer) and M'' (inner layer) corresponds to Ti, V, Nb, Ta, Cr or Mo. In the structure, C atom occupy octahedral position between M'-M'' layers. Each of the M' and M'' layer possess multiple surface termination groups such as F-, OH- etc. To validate DFT studies this group has synthesized Mo2TiC2Tx, Mo2Ti2C3Tx and Cr2TiC2Tx MXene and explored their electrochemical performance. This study further opens new choices in term of synthesis and applications of new-generation of MXene. Very recently Anasori, et al has discussed atomistic design of 2D transition metal carbides and nitrides. In this study, it has been also emphasized that structure of double transition metal MXene can be either ordered or can exist in random manner. In the case of ordered structure, the transition metals in MXene can be in-plane or out-of-plane (Figure 6). In random arrangements, the transition metals are dispersed as solid solutions (Figure 7). The differences in the structural arrangement in double transition metal MXene can lead to specific properties such as magnetic, optical, electrochemical, mechanical, catalytic and thermoelectric [6].
Figure 6: Di-transition metal MXene shows (a) in plane order (M′ 4/3M′′2/3X and M′4/3X), (b) out of plane order (M′2M′′X2 and M′2M′′2X3) and (c) solid solution of disordered MXene with M' (green balls) and M'' (purple balls) transition metals.
Figure 7: Crystal structures of five layered MAX and MXene (Adapted from Ref No. and reproduced with permission of copyright 2020 from American chemical society).
In a recent study, Gogotsi et al. has succeeded a scalable synthesis of Mo4VC4Tx which is a MXene with five atomic layers of two transition metals with twinning at central M layers. The PXRD pattern for the MAX (Mo4VAlC4) and the MXene (Mo4VC4) is presented in Figure). The insets in Figure show SEM images where the clear-cut presence of multilayer for Mo4VAlC4 (MAX) and MoVC4 (MXene) phases is observed. Indeed, in the MAX phase, the alternating layered structure with repeatedly darker layer after every sixth layer confirms that Mo4VC4 slabs are sandwiched between single Al layers (Figure 8). On the other hand, the Mo/V atomic layers are not sandwiched by Al layers, thus confirming the conversion from MAX to MXene (Figure 8(e) and 8(f)). Therefore, this study demonstrated the easy preparation of double metal transition MXene and especially the existence of several subfamilies like the present M5XTx, a MXene with a twinned structure. Indeed, a wide range of 2D MXene phases of varying compositions can lead to promising properties for potential applications [7].
Figure 8: PXRD patterns of Mo4VAlC4 (MAX), Mo4VC4 (MXene) powders and free-standing film of the delaminated MXene. Insets show SEM images of (a,b and c) MAX and (d,e and f) MXene (Adapted from Ref No. and reproduced with the permission of copyright 2020 from American chemical society).
Recent applications of MXene
This section is devoted to a detailed description of potential applications using MXene as versatile 2D materials based on their interesting structures, chemical compositions, type of surface terminations and thickness of the layers.
MXene as supercapacitor
MXene are found tobe a promising class of materials for electrochemical energy storage and electrocatalytic applications since they can be used in the form of an electrode. The performance of MXene as supercapacitor depends on the type of the electrolytes. Using ionic liquid or salt solution as electrolytes, the performance of MXene depends on their intercalation capacitance behavior, while for acidic electrolytes, it depends on their pseudo capacitance behavior. Though, the presence of surface termination groups in MXene structure makes their surface redox properties unclear. Indeed, the thermodynamic stability of MXene is relatively poor due to surface degradation in contact with air and water. The rate of this MXene oxidation depends on morphology, chemical composition and the employed etching method. Indeed, recent studies have pointed out that, although a colloidal suspension of Ti3C2Tx in an open vial appeared undisturbed for 15 days, appearance of white colour confirmed the formation of TiO2. Persson, et al. has mentioned that the HF etching treatment of Nb2AlC has an effect on the produced Nb2C MXene since the oxygen from the atmosphere is adsorbed, thus leading to the formation of clusters. The formation of clusters destabilized MXene structure and strongly altered negatively their properties, especially the electrochemical ones. Rizwan et al. has examined the nature of Nb-doped Ti3C2 MXene as energy storage material. The incorporation of Nb in Ti3C2 was made in order to enhance the ionic storage capacity and the stability of material. The PXRD pattern of doped sample is resembling with the undoped sample, only with small shift in 2θ positions towards lower angles and additional impurity lines of Nb2C and NbC phases (Figure 9). There is also a shift in the bandgap which is shifted to lower value for the doped sample. In addition, from the cyclic-voltammetry i.e., the I-V curve, a very high value of gravimetric capacitance of 442.7 F/g was calculated. This value of was approx. twice to the value reported for undoped Ti3C2 (245 F/g). Moreover, an excellent and boosted electrode performance can be seen in charge discharge triangular curve for Nb-doped Ti3C2 sample. Concerning the charge/discharge curve, the capacitance values were around 432.5 F/g up to 1,000 cycle and this value was acceptable up to 1,500 cycles but decreased to 306.8 F/g in the span of 1,600-2,000 cycle (Figure 10). A retaining trend observed in the specific capacitance is responsible to make it suitable for efficient electrode performance. Such high values of capacitance registered for the Nb-doped Ti3C2 make Nb-doped Ti3C2 MXene a good supercapacitor material [8].
Figure 9: (a) PXRD pattern and (b) bandgap estimation curve derived using UV-visible spectroscopy for Nb-doped Ti3C2 and undoped Ti3C2 MXene. (Adapted from Sugiura et al., and reproduced with permission of Copyright 2020 from Creative Commons Attribution License (CC BY).
Figure 10: (a) Graph of specific capacitance for Nb doped-Ti3C2 MXene, (b) Respective charge-discharge curve for cycle 1 and 2 and (c) specific capacitance V/s number of cycles (Adapted from Sugiura et al., and reproduced with permission of Copyright 2020 from Creative Commons Attribution License (CC BY).
For the first time, Grace et al. synthesized Ta4C3 MXene by HF etching of Ta4AlC3 MAX phase and shown as a promising material for energy storage applications. Besides this, Ti3C2Tx, Ti2CTx, Mo2CTx, Mo1.33CTx are MXenes exhibiting outstanding conducting behavior. These MXene also displayed pseudocapacitive behavior in charge storage and acting as potential electrode material too. Including larger surface area, high conductivity, larger interlayer spacing and high hydrophilicity; an abundant valance state of metal atom is very essential factor contributes towards high capacity of MXenebased supercapacitor electrode materials. Reported studies have shown that V-based V2CTx and V4C3Tx MXene exhibits variable oxidation states, that is responsible for high electrochemical performance in behaving as supercapacitor electrode material. Zhu et al. has confirmed the high specific capacitance of V4C3 MXene originates due to the variable oxidation state of MXene i.e., +2, +3, +4. Moreover, high-rate performance and high cyclic power was attributed to higher electronic conductivity of MXene.
Catalytic properties of MXene
The MXene possess interesting catalytic and photocatalytic properties for applications in water treatment and clean chemical energy. Photocatalysis is considered as a cost effective and environment friendly technology for environmental remediation along with the production of sustainable, renewable and clean chemical fuels. As per reported literature there are numerous semiconductor materials such as metal oxides, metal sulfides, nitrides, graphene and many of their composites that have been explored in photocatalytic applications [-]. Though, the current efficiency of traditional photocatalysts is quite limited to fulfill future demand, so alternatives are still wanted. Indeed, one of their limitations are the slow rate in photocatalytic reaction along with the incapability in scale up testing. More concretely, scientists need to improve (i) Ability of visible light absorption, (ii) Photo-induced separation and transport of charge carriers (from bulk to surface), (iii) Surface adsorption and redox properties and (iv) Stability of photocatalysts. To answer on, at least part of the existing problems, the MXene appear a promising candidate to design efficient photocatalytic composite materials. In addition, MXene can be used as adsorbent for the removal of organic pollution in water. Recently, Yoon et al. summarized the role of MXene and MXene-based materials as adsorbent for the removal of organic and inorganic contaminants from water. This study also discussed the factors which typically influence adsorption performance of MXene based materials. It not only includes physiochemical properties of the adsorbents but also cover impact of water quality such as pH value, temperature, background ion and Natural Organic Matter (NOM) over the adsorption capacity of MXene based materials. This study declared the following facts about adsorption of MXene-based materials: (i) Higher surface area with abundant functional groups, facilitates higher adsorption, (ii) With increasing pH value adsorption also increases due to more negative charge on surface, but at very high pH adsorption capacity decreases due to formation of hydroxides with targeted compound, (iii) higher the temperature more will be adsorption and (iv) presence of background ion and NOM affects selectivity of adsorbents for targeted contaminants. The results of recently reported studies which includes MXene-based photocatalysts and adsorbents in water treatment (removal of aqueous organic pollutants) are summarized in Table 3. Beside organic pollution, inorganic contaminants can be also removed using MXene-based materials. From the above compiled results in Table 3, it is clear that in order to behave MXene-based materials as photocatalysts, there should be a good utilization of light i.e., photon adsorption and good production of e-/h+ pairs, i.e., charge carrier separation along with high interfacial contact and superior redox ability. The photocatalytic and adsorption behaviour of MXene can be improved by carrying out doping at atomic level and also making hybrid or composite nanostructured materials. Overall analysis of the literature confirmed that MXene have promising applications in photocatalysis [9].
Table 3: Lists MXene-based photocatalysts and adsorbents in water treatment.
In addition to wastewater treatment, MXene-based materials have also attracted high attention of the researchers to explore them for H2 production and CO2 reduction under light. Nowadays, H2 is considered as a clean and sustainable energy fuel and is gaining great attention of researchers. However, the nowadays popular metal sulfides, metal oxides (e.g., TiO2, BaTiO3, Sr2Ta2O7 etc.) and g-C3N4 still contains considerable toxicity, fast recombination and/or low-light utilization capacity, thus are inefficient. Nevertheless, recent reports showed their combination with MXene as promising materials for H2 production by providing an intimate contact and Schottky junction between nanomaterials and MXenes (Table 4).
| Synthetic method | Disadvantage | Advantage |
| Fluoride etching | High toxicity of HF Lower HER activity |
Accordion-like structure Small flake size |
| Alkali etching | Formation of some oxide/hydroxides may further hinder the required Al extraction | Avoid the use of HF Remove the amphoteric or acidic atoms from original MAX phase |
| Electrochemical etching | Low yield | Avoid the use of HF Extend range of HF techniques and potential compositions of MXene Fabricate MXene difficult to prepare |
| Water-free etching | High temperature Low hydophilicity |
Expand the selection range of MXene precursor and MAX phase Provide a green and viable route to prepare MXenes |
Table 4: List of MXene-based photocatalysts in H2 production.
On the other hand, photocatalytic CO2 reduction into CH4, CO, CH3OH, HCHO and HCOOH is also another important study to solve greenhouse effect and energy storage. Nevertheless, due to low thermodynamic stability of CO2 molecules and their poor adsorption or activation over catalyst surface, the enhancement of existing photocatalysts is quite challenging. To overcome these issues, a combination of Ti3C2 MXene with TiO2 was reported by Low et al., where Ti3C2 act as co-catalyst. In this study, a high rate for CH4 production was observed with high stability of photocatalyst. The result of this study was supported by theoretical calculations and experimental results and concluded that ultrahigh conductivity and light absorption behavior of Ti3C2 was mainly responsible to the efficient charge carriers’ separation. Ever since this study, additional MXene-based materials were evalauted for CO2 reduction and the results are summarized in Table 5.
| Compositions | Sacrificial reagent | Light source | H2 production rate (µmol.g-1.h-1) |
| Ti3C2/TiO2 | Methanol | 200 W Hg (285ËλË325 nm) | 2650 |
| g-C3N4/Ti3C2 | Triethanolamine | 300 W Xe lamp with AM-1.5 filter | 5111.8 |
| MoS2/Ti3C2 | Methanol | 300 W Xe lamp (λË420 nm) | 6144.7 |
| MoxS/TiO2/Ti3C2 | Triethanolamine | 300 W Xe lamp (200ËλË1200 nm) | 10505.8 |
| WS2/TiO2/Ti3C2 | Triethanolamine | 300 W Xe lamp with AM-1.5 filter | 3409.8 |
| CdS/Ti3C2 | Lactic acid | 300 W Xe lamp (λË420 nm) | 2407 |
| CdS/MoS2/Ti3C2 | Na2S/Na2SO3 | 300 W Xe lamp (λË420 nm) | 9679 |
| CdLa2S4/Ti3C2 | Na2S/Na2SO3 | 300 W Xe lamp | 11182.4 |
Table 5: List of MXene-based photocatalysts in CO2 reduction.
The different combinations of 0D/2D, 1D/2D and 2D/2D structures are possible by the combination of different nanomaterials and 2D MXene materials, however, it is crucial to distinguish the interactions between the two materials. For instance, Su et al. synthesized MXene-based photocatalyst based on 0D P25 and 2D monolayer Ti3C2Tx, wherein P25 was dispersed uniformly over Ti3C2Tx monolayer Li et al. made a combination of 1D CdS nanowires and 2D nanosheets of Ti3C2 while He et al. prepared 2D Ti3C2 and 2D graphene. Recently, Zhang et al. has pointed out that there exist electrostatic interactions between positively charged semiconductor nanomaterials and negatively charged 2D MXene materials. This group also concluded that among 0D/2D,1D/2D and 2D/2D MXene-based photocatalysts, the 2D/2D ones exhibit larger specific surface area with greater interfacial contact compared to other structures. At last, Vasseghian et al. highlighted fascinating characteristics of magnetic-MXene-based nanocomposites and their extended behavior in various applications such as biosensors, cancer theragnostic, bioimaging and specially wastewater treatments.
Biomedical applications of MXene
The biomedical technology is one of the scientific fields which made a huge progress during the last decades. In biomedical applications, there is a high demand of new nanosized, non-toxic and/or luminescent materials. In this regard, MXene, e.g., Ti3CxOy and TiNxOy (and derived from Ti3AlC2 and Ti2AlN MAX phase, respectively) are promising due to biocompatibility and non-toxicity to living organism. Indeed, C and nitrogen are essential elements and early transition metal atoms like Ti are inert in biological organisms. The presence of functional groups (-OH, -O, -F) on the MXene surface are responsible for their hydrophilicity and thus their potential use in biomedical applications. Shi, et al. has shown that Nb2C nanosheets possess promising properties for in vivo photo-thermal ablation and eradication of tumor in NIR-1 and NIR-II bio-windows. Since MXene possesses strong absorption in NIR region, they are suitable for in vivo photoacoustic imaging. In this section, biosensors, antibacterial activity and bioimaging is discussed due to the interesting optical, magnetic and electronic properties of MXene.
Biosensors
In general, biosensors are self-contained integrating analytical devices that convert biological signal into quantifiable and processable signal. The most important features of biosensors are stability, sensitivity and reproducibility. There are several types of biosensors such as glucose monitoring biosensor, alcohol measuring biosensor, lactate biosensor, environmental pollution monitoring biosensors, immunoassay biosensors, Biological Oxygen Demand (BOD) biosensors. Based on theoretical and experimental studies, MXene-based materials (e.g., Ti3C2Tx, Ti3C2Tx/Au composite, Ti3C2 quantum dots, Ti3C2Tx/Nafion, etc.) intrinsically possess a high sensitivity for H2O2, NO2 - and small molecules such as glucose or phenol. Based on experimental facts, the study reported by Velic et al. claimed that Ti3C2Tx (T=-O, -OH) under cathodic potential is suitable for sensing purposes whereas under anodic potential range Ti3C2Tx forming TiO2 layer or TiO2 domain in aqueous solution. Indeed, it is a potential promising sensor for H2O2 with a detection limit of 0.7 nM which is enhanced compared to the reported highest value of 0.3 nM. Mahmoud et al. discussed the ultra-sensing role of Ti3C2Tx MXene-modified with glassy carbon, in sensing Bromate ions (BrO3-) with a linear detection limit of 41 nM. In addition, Yang et al. has noted that MXene including Ti3C2, Ti2C, Nb2C, V2C, Cr2C, Mo2C, Nb2N and Hf2C are suitable for sensing heavy metal ions such as Cd2+, Pb2+ and Cu2+ (with a detection limit of 0.1 μM) and Hg2+ (with a detection limit of 1.0 μM). Typically, this study recorded the electrochemical response of co-existing heavy metal ions (Cd2+, Pb2+, Cu2+ and Hg2+) with high sensing capacity, by employing alkaline-intercalated-Ti3C2 with unique surface chemistry. SEM images for pristine Ti3C2 and alkaline-intercalated Ti3C2 depicted layered morphology (Figure 11). In this study, Square Wave Anodic Stripping Voltametric (SWASV) method was used to identify trace concentrations of individual and co-existing heavy metal ions. The result of SWASV showed the following results: stripping behaviour for Pb2+ was in the range 0.10-0.55 μM with a detection limit of 0.032 μM and stripping behaviour for Cd2+ and Cu2+ was in the range from 0.1-1.0 mM and 0.1-1.4 mM with detection limit of 0.082 μM and 0.039 μM, respectively. For Hg2+, the stripping behaviour has lower value of sensitivity (1.0-1.9 μM) with a detection limit of 0.066 μM. Simultaneous detection of metal ions from their mutually co-existed solution was also performed using alkalineintercalated Ti3C2 and a clear separation of the peak for individual metal ion was observed with detection limit of 0.098 mM (Cd2+), 0.041 mM (Pb2+), 0.032 mM (Cu2+) and 0.130 mM (Hg2+), respectively. This study indicates that functionalized MXene are prone to be employed as sensing agents in tracing heavy metal ions towards environmental applications [9].
Figure 11: Schematic crystal structure representation for the stabilization of Alk-Ti3C2 from Ti3C2 (on top, left side), SEM images for Ti3C2 and Alk-Ti3C2 (on bottom left side) and SWASV curve for individual Pb2+, Cd2+, Cu2+, Hg2+, simultaneous analysis for mixture (Pb2+, Cd2+, Cu2+, Hg2+) and respective calibration curve for mixture.
Intracellular pH sensing is another important aspect in biomedical science since it helps to monitor the evolution of various disease and health of cells where pH is a relevant indicator. Recently, Song, et al. has demonstrated that the luminescence of Ti3C2 quantum dots is pH sensitive due to the protonation/deprotonation of the functional group.
Antibacterial activity
Antibacterial feature is quite complex since it includes a living organism whose life is affected by toxic substance. Photoinduced activity of semiconductor photocatalysts is one of the most investigated technologies for antibacterial application, due to the generation of electrons and holes that act directly or indirectly as toxic substances for the bacteria. In order to get advancement in this field, MXene have been tested for antibacterial activities since they use electron from F-subshell of transition metal atom to shift reactive electrons to the cell membrane and thus can be potentially used in type-I photodynamic therapy like cell killing. Gogotsi, et al. examined the antibacterial activity of Ti3C2 in killing Escherichia Coli and Bacillus Subtilis cells (Figure 12). In this study, the bacterial cells were cultured in the presence of Ti3C2 for 4 hours to examine inhibition growth the cells. The results in Figure exhibits clearly an effect of the MXene material since the number of colonies significantly decreases with increasing the concentration of MXene. The mechanism proposal of antimicrobial activity is the hydrogen bonding between oxygenated groups at the surface of Ti3C2 MXene and lipopolysaccharide strings of cell membrane, thus preventing intake of nutrients. As noticed, 2D MXene possess higher efficiency in inhibiting bacterial growth than graphene oxide nanosheets due to higher conductivity.
Figure 12: The reaction scheme for the antibacterial activity of Ti3C2Tx MXene (a) and images of “E. coli.” bacterial cells on the agar plates with different concentrations of MXene (b), respectively and reproduced with the permission of copyright 2018 from American chemical society).
Bioimaging
Bioimaging is a complicated process of acquiring, processing and visualizing functional image of living objects. It includes Computed Tomography (CT), Magnetic Resonance Imaging (MRI) and other imaging techniques. These techniques create images of human body, anatomical area, tissues, etc., for clinical purposes. In this type of biomedical application, MXene are suitable as contrasting agent in bioimaging. Indeed, due to the semimetal behaviour of MXene, a strong photothermal effect by absorption of light that is subsequently converted into crystal vibrations is exhibited, thus releasing energy in the form of heat. Several scientists investigated different MXene, e.g., Ti3C2, Nb2C, Ta4C3 for in vivo Plasminogen Activator Inhibitor (PAI). In addition, Zhi, et al. noticed that MXene Quantum Dots (MQDs) possess luminescent properties. These MQDs were obtained by fabricating Ti3C2 under solvothermal conditions at different temperature conditions such as 100°C, 120°C, 150°C and symbolized as MOD-100, MQD-120 and MQD-150 in Figure. According to TEM analysis, the average particle size was found to be 2.9 nm (for MQD-100), 3.7 nm (for MQD-120) and 6.2 nm (for MQD-150) while the MQD-100 sample is retaining structure as that of Ti3C2 MXene, but on raising temperature, the structure got ruptured. Owing to small Eg of Ti3C2, it is quite reasonable to increase it by quantum confinement effect and also to achieve interesting luminescence behaviour. In the UV-visible absorption spectra of MQDs, two peaks were observed at 260 and 320 nm and similarly, two peaks were observed at 250 and 320 nm in photoluminescence excitation spectra. It has been also observed that MQDs has excitation dependent photo-luminescent behaviour and that with an increase in excitation wavelength from 330-440 nm there is a shift in PL spectra from blue to red. The estimated quantum yields values were found to be higher for MQD-100 (9.9%) and MQD-120 (8.7%) samples than MQD-150 (7.9%). So, these high values of quantum yield suggested a potential use of MQDs as a reagent in multicolour cellular imaging and optical material too. Consequently, this study demonstrated that MQDs has huge importance in optical, biomedical and environmental applications.
MXene as magnetic materials
In general, magnetic materials have various applications potential such as, informatics, telecommunications, actuators, sensors, motors, generators, etc. Though, when MXene are combined with magnetic nanomaterials, the resulting nanocomposites possess interesting behavior in energy and environmental applications. There are also MXene that exhibit magnetism like those prepared from ferromagnetic and paramagnetic MAX phases like Mn2GaC, Cr2GeC, (V, Mn)3GaC, (Mo, Mn)2GaC and (Cr, Mn)2AlC. Wang, et al. has discussed the magnetic properties of Cr-based MAX phases. In this study, magnetic state of (Cr2Ti)AlC2 and Cr2AlC are due to the net spin of Cr3d valence electrons. The other atoms in MAX compounds have negligible contribution to the magnetic moment. The observed magnetic moment of (Cr2Ti)AlC2 was found to be higher than Cr2AlC, since in there is a significant distance between out plane of Cr which are separated by Ti-C slabs. In MXene, the magnetism arises from (i) Intrinsic properties of transition metal in the material, (ii) Defects associated with 2D layer or (iii) Surface functionalization. Xue et al. has carried out theoretical calculations based on density functional theory in order to know the magnetic properties of M2C, where M=Hf, Nb, Ta, Sc, Ti, V and Zr. This study reported that only Ti2C and Zr2C exhibits magnetic behavior with magnetic moment of 1.92 and 1.25 μB/unit at zero strain state. But on increasing tensile strain, it has been demonstrated that the other M2C MXene exhibit a transition from nonmagnetism to ferromagnetism. P. Nachtigall, et al. has investigated the magnetic behavior of Mn2CT2, where T=F, Cl, O, OH, H on the basis of computational studies. This study examined that Mn2CT2 are the only MXene having ferromagnetic ground state. The Mn2C retains its ferromagnetic behavior when it is symmetrically functionalized with functional groups bearing a -1 charge (for example F, Cl and OH). This study provided evidences that, on changing the functional group from F- to I-, there will be only a quantitative change in the property of Mn2CT2. Though on changing functional group from O2- to H+, there will be a qualitative change in magnetic properties (ferromagnetic semi-metal to antiferromagnetic metal or semiconductor). Therefore, results of this study claimed Mn2CT2 MXene have good application in spintronic. P. Nachtigall, et al. has also elaborated on asymmetrical functionalization of Cr2CFCl, Cr2CClBr, Cr2CHCl, Cr2CHF and Cr2CFOH which exhibit magnetic behavior with a high Neel’s temperature (400 K). Scheibe, et al. has explored the magnetic properties of Ti3C2Tx with different functional groups. In this study Ti3C2Tx derived by using HF and Chlorosulfonic (CS) acids exhibited paramagnetic behavior only, whereas MXene derived by employing mixed acids revealed mixed paramagnetic/antiferromagnetic behavior at an applied external field of 100 Oe.
Rizwan, et al. has carried out a comprehensive study on computational and experimental analysis of magnetic behavior of Nb-doped Ti3C2, since separately Nb2C is diamagnetic and Ti3C2 is ferromagnetic. In his study, DFT calculations has shown an increased in c-lattice parameter from 19.2 Å to 23.4 Å on Nbdoping and demonstrated ferromagnetic behavior for both Nbdoped as well as pristine Ti3C2 samples. PXRD results exhibits a good resemblance with theoretical investigations. Indeed, as per existing literature, its ferromagnetism is highly unstable in nature, so when it is doped with Nb, it remains ferromagnetic. The M-H curve for Nb-doped Ti3C2 shows pure ferromagnetic behavior of the sample at 100, 200 and 300K temperature). A high value of saturation magnetization was observed for the sample at 300 K that might be due to the presence unpaired spin of niobium or its oxide impurities. The crystal structure of pristine and Nb-doped Ti3C2 is presented). These experimental studies have confirmed an increased ferromagnetic behavior in Nb-doped Ti3C2 sample than pristine sample [10].
MXene as gas sensor
Gas sensors are devices that monitor the presence of gases and as well as quantify them. Presently, sensors play a crucial role in monitoring air quality, controlling air pollution, diagnosis, therapeutics and breathe monitoring. On the basis of working principles, gas sensors are categorized as infrared sensors, ultrasonic sensors, electrochemical sensors and chemiresistive sensors. Due to simple configuration, low cost and relatively high accuracy, chemiresistive sensors are of high interest. The chemiresistive are working on the principal of a chemical reaction taking place between sensors surface and introduced gases. In order to behave as a promising sensor, the sensing materials should have high surfaced area, active surface sites for effective, selective adsorption of gas molecules and should have high ability to transfer chemical interaction into electrical signal. Since past decades, 2D materials have gained much interest as sensor materials due to their high surface area. Though there are many 2D materials which are having good performance as sensor but still they are having some issues such as the operating conditions (high temperature if often required), low electrical conductivity and limited sensitivity. Thereby, to overcome these issues, MXene are developed as next generation sensors. Although the utilization of MXene as gas sensors till date is quite limited, MXene serves as best platform to develop different types of sensors. Indeed, MXene exhibit high surface conductivity, enough terminal functional groups, high hydrophilic nature and robust mechanical properties. Reported literature suggests that different compositions of 2D MXene displayed significant activity in sensing various gases such as NH3, H2, CH4, CO, CO2, N2, NO2 and O2. Xiao, et al. has shown that M2C (M=early transition metal) has high selectivity to sense NH3 gas over other gases. Additionally, the adsorption of CH4, CO2, CO, NH3, H2, N2, NO2, O2 also have been investigated by utilizing Ti2CO2 MXene. This study emphasizes on the fact that with increasing applied strain, the adsorption of NH3 over Ti2CO2 surface can be increased possibly. Though, adsorption of other gas is week at same applied strain which further confirmed that Ti2CO2 is potential sensor for NH3 adsorption. This study also noted that adsorption of NH3 is a reversible process, since on releasing applied strain NH3 will be desorbed from Ti2CO2 surface.
Ma et al. has investigated the potential behaviour of Ofunctionalized MXene i.e., M2CO2 (M=Sc, Hf, Zr and Ti) for adsorption of highly toxic SO2 gas and found them highly reliable and efficient. Stanciu et al. has described the capability of surface functionalized Ti3C2Tx towards the sensing of Volatile Organic Carbon (VOC) compounds. In this study, Ti3C2Tx has been found a very efficient sensor of ethanol and acetone. Moreover, Pasha et al. has summarized the sensing properties of MXene and their composites for different VOCs present in breath. The VOCs included in breath are ammonia, acids, alcohols, aldehydes, ketone while non-VOCs includes hydrogen, nitrogen dioxide, carbon dioxide and sulfur dioxide gases. Basically, sensing of VOCs and non-VOCs can provide a lot of information related to metabolic problem occurring in human body. This study justified that MXene can detect various VOCs since a solid interaction (i.e., hydrogen bonding) takes place between gas molecules and surface terminated groups of MXene (-O and -OH). The hydrogen bonding facilitates transfer of charge carriers from adsorbent to adsorbate gas. This study also confirmed that on introducing defects and surface sensitization in MXene materials, there is an increase in number of surfaceactive sites and charge carrier concentration which offer wide range of sensing applications. The interesting properties of MXene such as high surface area, room temperature detection and easy to assemble, lead to a better sensing device over the reported one and it has been proven on the basis of theoretical and experimentally investigation.
MXene as thermoelectric power generation
The generation of electricity by utilizing waste is an important area of research and fall under the category of thermoelectric technology. The sources for this waste (thermal energy) are nuclear power plants, automobiles, thermal power plant, natural gas, glass and aluminum factories etc. The device used to convert direct thermal energy into electric energy are referred as thermoelectric generators and working on the principle of Seebeck effect. Basically, thermoelectric materials have been divided into three categories based on their optimal working temperature. It involves Bi2Te3-based (below 500 K), PbTe-based (between 500-900 K) and SiGe-based (above 900 K) materials. Though, there is need of high efficiency of thermoelectric power generators which require good thermoelectric material i.e., materials with high value of dimensionless-figure of merits (ZT). According to theoretical studies, it has been predicted that most of the MXene are metallic in nature except a few of them like ScC2Tx, Ti2CTx, Zr2CTx and Hf2CTx which have a non-zero band gap. Therefore, such semiconducting MXene are supposed to have high value of Seebeck coefficient e.g. 1140 μV/K for Ti2CO2 and 2200 μV/K for Sc2C(OH)2. Sakka et al. has mentioned Mo2CF2 as a superior thermoelectric material (based on theoretical calculations). Though, these calculations were made in assumption of fully controlled surface terminations while experimentally, it is quite challenging since multifunctional group coexist at the surface of MXene. Hope, et al. has demonstrated that the nature of surface termination groups depends on synthetic methods. Concerning mono transition msssetal MXene, they exhibit thermoelectric behavior with maximum ZT value of 1.1, thus exploring double ransition metal MXene for thermoelectric properties is a hot area of ongoing research. Alshareef, et al. has synthesized three different compositions of MXene i.e., Mo2CTx, Mo2TiC2Tx and Mo2Ti2C3Tx. In his study, it has been observed that these MXene exhibits a high value of electrical conductivity with ntype Seebeck coefficient. Among these three MXene compositions, Mo2TiC2Tx has the highest electrical conductivity, largest Seebeck coefficient with thermoelectric power of 3.09 × 10-4 W/mK at 803 K. Recently, Cheng, et al. has reported the thermoelectric behavior of Cr2TiC2 and CrTiC2T2 (T=F- and OH-) with a high value of Seebeck coefficients i.e., >800 μ V/K and >700 μ V/K, respectively. Figure 18 demonstrates on the ZT values these double transition metal MXene. As per the reported studies a high value of ZT, approx 3.0 at 600 K, are observed which are significantly higher than the reported ZT values for other well-known thermoelectric materials. Thereby, overall, it can be concluded that MXenes are found to be promising material for high efficiency of thermoelectric power generators.
Miscellaneous
In previous years, 2D materials have gained the attention of researchers due to quantum confinement of thin layers. The properties of 2D materials strongly depends on composition and thickness of materials. Typically, their electronic and optical behavior is dependent to elemental doping, strain engineering and applied external field. Investigations of MXene as 2D materials in 2011 by Gogotsi, et al. was a new addition in the series of existing 2D materials and bring revolution in the field of 2D materials. On the basis of theoretical studies, different compositions of MXene have been identified to behave as topological insulator i.e., quantum spin Hall effect, metal-toinsulator transition, superconductors etc., but still it has not been proved experimentally much. Till date, the fundamental properties such as their intrinsic electronic transport, interaction with electromagnetic waves and mechanical behavior of MXene is still not clear. Though, reported studies have shown that a manageable band gap, high electrical conductivity, high elastic moduli and ability to intercalate ions and good compatibility behavior of MXene make them significantly important for flexible transparent electrode applications. Moreover, the reflecting behavior in UV region offers to utilize MXene materials as an anti-ultraviolet ray coating material. Recent DFT studies have shown that structural and electronic properties of MXene are altered by surface functionalization.
The occurrence of surface termination causes variation in structural properties as well as stability of MXene. Reported studies have shown that metallic MXene become semiconductor after surface functionalization. Basically, functionalization of MXene with surface termination groups O, F and OH depends on synthesis method of MXene. Majorly linear and non-linear properties of MXene are strongly depends on their electronic structure. The linear properties include absorption and photoluminescence behavior while non-linear properties are considered as refractive index or saturable absorption etc. In earlier reported studies, optical properties of various MXene (e.g. Ti2C, Ti2N, Ti3C2 and Ti3N2) have been examined by using Random Phase Approximation (RPA) method. These materials are possessing good metallic conductivity which facilitates them to transmit electromagnetic waves. The presence of number of layers in MXene also affects their optical properties. Though, termination of surface group strongly alters their electronic structure. It has also observed that Ti3C2 have smaller value of absorption coefficient than the pristine sample existing without termination of F- and OH- surface groups. Thereby by controlling surface functionalization, optical properties of the MXenes can be tuned [11].
In modern technology, MXene have become an eye catching 2D material. To the best of our knowledge, MXene have well defined properties such as half metallicity, hydrophilicity and good electrical conductivity that make them suitable in wide range of applications. In the last decade, MXene family has been explored quite a lot and it is growing day by day more and more. The current review emphasizes mainly on processing, engineering and performance of MXene materials. In terms of processing, there are various MAX and Non-MAX phases serving as precursors, that can be subjected to different synthetic conditions in order to achieve targeted MXene materials. In addition to monometallic MXene, new discoveries are focusing on engineering of double transition metal MXene as well as fluoride free. Subsequently, MXene structures are accessible in different morphologies from monolayer to multilayer, that also depend on synthetic route and precursor materials. This different dimensionality nature of MXene leads to alteration of their physical and chemical properties, which further nourishment their exploration for energy applications, environmental applications, but also in catalysis, sensing and biomedical applications. Though, study on MXene is still quite limited and to further enhanced their development, the following research directions might be considered:
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Atri S, Domanska MM, Motola M, Plesch G, Monfort O (2025) MXene as Versatile 2D Materials: A Review of the Recent Progress in Synthesis and Applications. J Chem Eng Process Technol. 16:543.
Received: 03-Aug-2024, Manuscript No. jcept-24-33352; Editor assigned: 07-Aug-2024, Pre QC No. jcept-24-33352 (R); Reviewed: 21-Aug-2024, QC No. jcept-24-33352; Revised: 03-Jun-2025, Manuscript No. jcept-24-33352 (R); Published: 10-Jun-2025 , DOI: 10.35248/2157-7048.25.16.543
Copyright: © 2025 Atri S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.