
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Mini Review - (2023)Volume 13, Issue 6
Mustard Oil Cake (MOC) is a common bio fertilizer used widely in fish cultivation in almost all tropical countries. In sublethal concentration, the level of liver protein and lipid, hematological parameters, as well as brain Cholinesterase (ChE) activity of fish were affected due to the stress of this biofertilizer. Though C. punctatus has the ability to tolerate stressful situations, it is better to use MOC carefully during the preparation of the pond rather than direct application in the water for better production of the fish.
Oil cake; Protein; Lipid; Blood; Cholinesterase
The demand for aquatic products has increased significantly during recent decades internationally. Due to the increase in the climate crisis, biodiversity loss, and the recent pandemic, the target of making a hunger-free world is in an adverse situation. Along with the agro-food, aquatic food is also recognized as key food security and nutrition for the people globally. A total of 7% of animal protein intake daily by humans is coming from aquatic food, worldwide. The demand for aquatic products has increased significantly during the recent decades [1]. To reduce aquatic pollution, and stop aquatic biodiversity loss, attempts are made to use more organic fertilizers and organic pesticides for effective management of all fisheries and maintaining the food chain in the aquatic ecosystems. Mustard Oil Cake (MOC), an organic fertilizer, is used in pond fishery frequently. The study revealed that MOC could be utilized at 30% to 35% composition in the diet as a protein source for different herbivorous fishes including carp. Such application did not hamper the fish growth [2-4]. For the increase in production of fish, suitable and cheap locally available fish feed is required. The most Potential substitutes to fish meal in carp diets are meals containing oil seeds like MOC, linseed, and sesame oil, besides soya bean meal [5,6]. Application of the right amount of protein-rich MOC with rice bran, and vegetable wastes showed an increase in the production of fish [7,8]. Fishes are an important staple food in a large part of the world due to the presence of ample amounts of lipids and proteins. Any effects of the xenobiotics on fish are devastating to humans [9]. Fish could respond to various biochemical and physiological strains that are secondary stress responses in comparison to the higher vertebrates [10]. Fish could recover from the stress in most cases [11]. Discreet use of biofertilizers in ponds increases productivity by producing autotrophs. Phytoplankton and algal populations increase in both rearing and stocking ponds [12-15]. However, it is essential to understand the effects on fish if they come in direct contact with MOC in the pond.
The work is conducted on the basis of previous research on the effect of MOC on fish. Different research papers and reports are considered to prepare this review.
Importance of MOC in fish farming
MOC is considered an important biofertilizer for the fish culture. 60% of the seed is converted into a byproduct as MOC during oil extraction [16]. It is used in preparing the ponds before the release of the fingerlings in the ponds. MOC contains 43% protein, 2.05% oil, 1.22% allylisothiocyanate, and 2.75% phytic acid with a considerable proportion of albumin, glutelin, and globulin [17,18]. The protein is rich in lysine and sulphurcontaining amino acids and is considered a protein supplement for human nutrition.
Effects on protein and lipid content
When C. punctatus was exposed to water treated with sublethal doses of MOC, no mortality was observed after 35 days. Initially, the rate of increase of Total Liver Protein (TLP) was very slow, but a steady rate of increase of TLP was observed. Strength of association (ῳ2) is estimated to measure the degree of relatedness between duration of Exposure (EP) and liver protein concentration. The computed values are 0.99 (EP=35)>0.95 (EP=07)>0.94 (EP=28)>0.82 (EP=21)>0.47 (EP=04)>0.38 (EP=14) (Table 1).
| Statistics | Day 4 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 |
|---|---|---|---|---|---|---|
| F* | 19.87 | 399.59 | 13.64 | 96.17 | 309.01 | 521.39 |
| ῳ2 | 0.47 | 0.95 | 0.38 | 0.82 | 0.94 | 0.99 |
| t-test† | 4.45 | 1998 | 3.69 | 9.8 | 17.57 | 72.21 |
| Bonferroni modification | P<0.002 | P<0.002 | P<0.002 | P<0.002 | P<0.002 | P<0.002 |
Note: *significant p<0.05; †significant p<0.001.
Table 1: Relations between control and MOC treated C. punctatus [19].
When C. punctatus was exposed to different doses of MOC, the liver showed a stress response initially. The liver showed an increase in sinusoidal space and lipidosis at its early exposure stage, followed by a recovery from the stress of MOC on the 28th day (Figure 1). After the initial setback, the growth rate i.e., weight, length, and breadth gradually increased from the 7th day, and weight gain was 9.64% on the 28th day. The muscle protein, Bone Morphogenetic Protein (BMP) of the fish also showed a similar trend. BMP was increased with the advance of days of exposure.
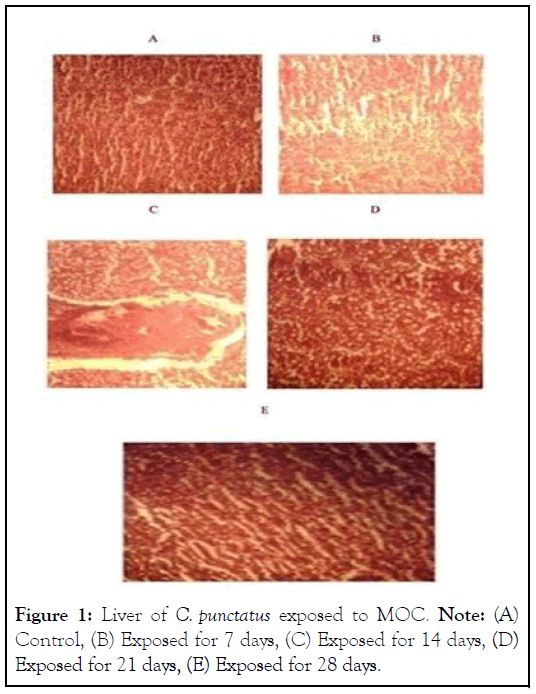
Figure 1: Liver of C. punctatus exposed to MOC. Note: (A) Control, (B) Exposed for 7 days, (C) Exposed for 14 days, (D) Exposed for 21 days, (E) Exposed for 28 days.
Effects on haematological parameters
The Total Count (TC) of RBC in non-treated and MOC-treated C. punctatus was 3.926 ± 7.65 106 mm-3 and 3.068 ± 1.5 106 mm-3 respectively after 4 days of exposure. But TC increased to 7.058 ± 7.22 106 mm-3 and 2.588 ± 4.01 106 mm-3 respectively after 28 days of exposure, which indicates a decrease in treated fish. Similarly, hemoglobin was 8.14 ± 0.2% and 7.44 ± 0.05%. On 4th day in non-treated and treated fish, which increases to 12.06 ± 0.09% in non-treated and 4.94 ± 0.09% in treated fish after 28 days. Differential Count (DC) of lymphocytes and neutrophils was found to increase after 28 days of exposure (Table 2).
| Leucocytes | 4 Days | 7 Days | 14 Days | 21 Days | 28 Days | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | T | C | T | C | T | C | T | C | T | |
| Lymphocytes | 38 | 38 | 38 | 39 | 38 | 41 | 39 | 39 | 39 | 43 |
| Eosinophils | 10 | 9 | 10 | 10 | 12 | 10 | 12 | 11 | 13 | 9 |
| Monocytes | 17 | 17 | 16 | 15 | 13 | 13 | 10 | 10 | 9 | 14 |
| Neutrophils | 6 | 5 | 7 | 6 | 9 | 9 | 11 | 11 | 11 | 12 |
| Basophils | 16 | 16 | 15 | 15 | 11 | 11 | 8 | 9 | 6 | 9 |
| Heterophils | 9 | 10 | 9 | 10 | 11 | 10 | 12 | 12 | 13 | 7 |
| Thrombocytes | 4 | 5 | 5 | 5 | 6 | 6 | 8 | 8 | 9 | 6 |
Note: C: Control; T: MOC-Treated.
Table 2: Differential count (%) showing variations in Control (C) and, MOC-Treated (T) C. punctatus.
Brain Cholinesterase (ChE) activity
ChE activity and body length have a significant correlation in C. punctatus, ChE activity has proportionality with the cell surface and varies with the body size of the fish. An initial increase in ChE activity in MOC-treated fish showed the tolerance of this animal against the effect of the biofertilizer used. Then ChE activity decreased, followed by an increase after 21 days and maintained up to 35 days in comparison to non-treated fishes. That was probably due to overcoming the capacity of the fish to survive in stressful situations.
MOC is rich in protein. The liver increases the synthesis of protein during prolonged exposure after overcoming the initial low rate of increase. The quantity of protein in the body depends upon the synthesis or degradation of proteins. Improper incorporation of the amino acids in the polypeptides also affects the quantity of proteins [20]. The protein level in the body also decreases due to the inhibitory activity of alkaline phosphatase [21]. The increase in the protein level in the body muscle indicates the fish's ability to compete with the initial opposing stress situation. This is due to more protein being used from the MOC of the medium to meet the increase in energy demand, and protein synthesis increases [22].
Survival mainly depends on the protein synthetic potential of animals [23]. An initial decrease in muscle and liver protein indicates an increase in proteolytic activity that is probably due to an increase in catabolic activity and a decrease in the anabolism of protein [24,25]. Such a response of fish is known as a secondary stress response in contrast to higher vertebrates [10]. Hepatic lipid level was found to decrease after 21 days of exposure in C. punctatus. Lipids provide energy in the metabolic process and energy reservoir of the body. Decreased liver lipid indicates the utilization of stored lipids to mitigate the demand during stressful situations [26]. However, liver lipid levels after 35 days indicate the recovery from the MOC effect [27]. Any variation in lipid profile will cause structural change in the cell membrane [28]. Any alteration of the chemical composition of the natural environment affects the physiological system of the aquatic inhabitants including fish [29,30].
Reduced TC of RBC was probably due to degeneration of erythropoietic tissue in the body or may be due to haemodilution [31,32]. Such reduction of RBC also reduces the Hb percentage in the blood. Solomon, et al. [33], also observed noticeable changes in haematological parameters in C. striata indicating slow recovery from the disadvantageous condition for fish. A gradual decrease in erythrocyte count and Hb indicates anaemia because of the breakdown of RBC due to the influx of MOC into erythrocytes as was observed in urea-treated H. fossilis [11]. MOC contains Allyl Isothiocyanate (AITA) and phytic acid. AITA helps the plant to defend itself from herbivores and phytic acid chelates multivalent metal ions like iron. Salt is produced which does not absorb properly from the intestine and the body suffers from the availability of minerals [34]. The decreased level of haemoglobin in MOC-treated fish is also probably due to less availability of iron. Variation of MCH in MOC-treated fish is probably due to swelling of erythrocytes or release of young RBC containing less haemoglobin in the blood circulation [35-37].
A decrease in the ChE activity in fish exposed to xenobiotics is not suitable for survival. 70 to 80% inhibition of ChE is considered to be critical for fishes [38,39]. C. punctatus has the ability to tolerate MOC as was observed in organophosphatetreated fish and such fast recovery may be due to the continuous synthesis of essential enzymes [40-43].
The study revealed that the application of Mustard Oil Cake (MOC) has a significant stress-inducing impact on C. punctatus. So, direct application in the pond water is not advisable, because of its impact on the different physiological parameters on fish bodies. Though C. punctatus has the ability to recover from stressful situations, but scrupulous use of MOC during the preparation of ponds for fish culture may increase the production, provided that its potential adverse effects on the fish's well-being are appropriately managed and monitored.
The author is thankful to the principal, Dr. Santanu Chakrabarti, Government General Degree College Singur, and Head of the Department of Zoology Dr. Debojyoti Chakraborty, of the same college for encouragement during the writing of this manuscript.
The authors declare no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Nath S (2023) Mustard Oil Cake (MOC) an Organic Fertilizer, its Toxicity and Response of Channa punctatus: A Review. J Clin Toxicol. 13:548.
Received: 30-Oct-2023, Manuscript No. JCT-23-27285; Editor assigned: 05-Oct-2023, Pre QC No. JCT-23-27285 (PQ); Reviewed: 19-Oct-2023, QC No. JCT-23-27285; Revised: 26-Oct-2023, Manuscript No. JCT-23-27285 (R); Published: 02-Nov-2023 , DOI: 10.35248/2161-0495.23.13.548
Copyright: © 2023 Nath S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.