Journal of Infectious Diseases & Preventive Medicine
Open Access
ISSN: 2329-8731
ISSN: 2329-8731
Review Article - (2021)Volume 9, Issue 11
Phlebotominae of sandflies are the vector of leishmaniasis, a disease that spreads to more than 98 countries worldwide.Visceral leshmaniasis is a neglected tropical disease caused by Leishmania spp, a protozoan parasite that can be transmitted by the bite of a sand fly infected Phlebotomus spp in the old world and Lutzomyia spp in the new world. Since the introduction of synthetic chemical insecticides in the 1940s, they continue to be an effective tool for controlling insects that carry disease pathogens. Unfortunately, insecticides are used indiscriminately and tremendous selective pressure is applied to resist insecticides. Although most species of sandflies are exposed to all major insecticide groups in the world, further evidence suggests that some Phlebotomine sandflies may be developing insecticide resistance. Some populations of sandflies are tolerant or resistant to insecticides used in the Middle East, South Asia, and South America. Such as DDT, Phlebotomus argentipes resistant to Pyrethroids from different regions of India reported. To provide authentic information about this novel, the reliable data on academic resources such as Google Scholar, Scopus, Web of Science, Springer, Pro-Quest, Wiley Online, Science Direct, Research Gate, PubMed, Sage, and SID were used. Different levels of susceptibility to insecticides have been reported around the world. A review of literature on sand fly susceptibility in Southeast Asia shows that P Phlebotomus argentipes, the main vector of VL, have shown resistance to DDT. Insecticide resistance has not yet been proven in Lutzomyia longipalpis but there are some signs of its occurrence in this species. For up-to-date information on vector susceptibility to insecticides, periodic monitoring of insecticides should be performed for susceptibility testing. Irrational longterm use of insecticides may cause tolerance or resistance to the target insects. To control the resistance to insecticides in sand flies and other VL and CL vectors, the use of rotation, mosaic and insecticide mixtures are possible methods. Furthermore, guidelines are needed for monitoring and evaluation of insecticide susceptibility tests against sand flies.
Insecticide resistance; Susceptibility; Vector; Visceral leishmaniasis
Ad: Adlerius; CL: Cutaneous Leishmaniasis; DL: Diffuse or Dermal Leishmanoid Leishmaniasis; Eu: Eu Phlebotomus; L: Leishmania; La: Larroussius; Lu: Lutzomyia; Med: Mediterranean; P: Phlebotomus; Pa: Para Phlebotomus; Pf: Pifanomyia; Sy: Syn Phlebotomus; VL: Visceral Leishmaniasis.
Background information
Phlebotominae of sandflies is a vector of leishmaniasis, a disease that spreads to more than 98 countries worldwide. Cutaneous Leishmaniasis (CL), Mucocutaneous Leishmaniasis (MCL), and VL or "kala-azar" are the three main clinical forms of the disease. Leishmaniasis is more common in tropical and temperate regions where sandflies are more common. The disease is endemic in 88 world countries (Figure 1) [1-3].
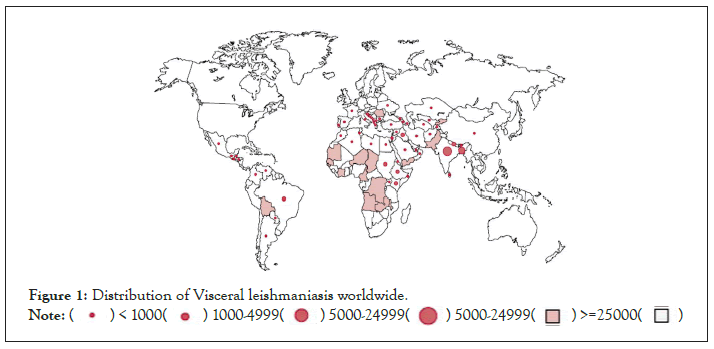
Figure 1: Distribution of Visceral leishmaniasis worldwide.
Visceral leishmaniasis, or kala-azar, is a neglected tropical disease caused by Leishmania spp, a protozoan parasite that can be transmitted by the bite of a sand fly infected Phlebotomus spp in the old world and Lutzomyia spp in the new world (Table 1) [4] and dogs are the main reservoir (Figure 2).
| Parasites | Disease | Countries | Reservoir | Incriminated vector | Suspected vector |
|---|---|---|---|---|---|
| L. donovani | VL, DL, CL | Northeast India, Nepal, Bangladesh, (Bhutan), Sri Lanka | Human anthroponosis | Ph. argentipes | None |
| L. donovani | VL | People’s Republic of China | Unknown | None | Ph. alexandri; P. (Ad.) species |
| L. donovani | VL, DL | Sudan, Ethiopia, (Chad), (Yemen) | Human anthroponosis; possibly mongoose? | Ph. orientalis | None |
| L. donovani | VL, DL | Sudan, Ethiopia, Kenya, (Uganda) | Human anthroponosis? | Ph. martini | Ph. celiae, Ph.vansomerenae |
| L. infantum | VL, CL | Med Europe, North Africa, Southwest Asia, People’s Republic of China | Domestic dog, wild canids, domestic cat | P. (La.) ariasi, perniciosus | P. (La.) species; P.(Ad.) species |
| L. infantum | VL, CL | Latin America: not Peru or Guianas | Domestic dog, wild canids | Lu. (L.) longipalpis | Lu. Species; Lu. evansi |
Table 1: Disease types of Visceral leishmaniasis worldwide.
Figure 2: Infected dog with Visceral leishmaniasis.
After malaria, this disease is the most common cause of death due to parasitic diseases. The disease is mainly distributed in East Africa, South Asia, South America, and the Mediterranean region. It is estimated that about 50,000 to 90,000 new cases of Visceral leishmaniasis occur each year [5]. More than 90% of new cases of Visceral leishmaniasis are reported from six countries: Bangladesh, Brazil, Ethiopia, India, and South Sudan and, Sudan [4].
In Asia, Leishmania donovani is the causative agent. There is no known animal reservoir (human transmission). East India (Bihar) and Bangladesh (Mymensingh District) most endemic areas, followed by Nepal In Southeast Asia, the distribution of VL in Bangladesh, Bhutan, India, Nepal, Sri Lanka, and Thailand is limited [2, 4]. Ph. argentipes (Diptera: Psychodidae) is a major vector in the Southeast Asia region [6]. Ph. argentipes usually rest indoors in cowsheds, human houses, and mixed human-cow houses, while outdoor resting has also been reported in tree holes and underwater.
In Africa, VL occurs in Sudan, South Sudan, Ethiopia, Kenya, Uganda, and Somalia. Here, is Leishmania donovani, but this is very different from that found in Asia. Human-human-animal transmission is mixed, although the exact reservoirs of the animal and its share have not been determined [4]. The Phlebotomus (Larroussius) orientalis and Phlebotomus (Syn Phlebotomus) are incriminated vectors in two distinct bioclimatic regions of East Africa [7].
In the Mediterranean, VL is caused by L. infantum and all the countries of the Mediterranean are endemic. Dogs are known reservoirs. Other indigenous regions include China, Sri Lanka, Thailand, Bhutan, various Central Asian countries, Iran, Iraq, and Saudi Arabia. The L. infantum and L. donovani are found, and dogs, jackals, and foxes are reservoirs. In Central and South America, VL is caused by L. infantum and dogs are the main reservoirs [4]. The Lutzomyia longipalpis (Diptera: Psychodidae) is the most important vector of L. infantum, a parasite that causes common human-animal Visceral leishmaniasis in the Americas [8]. Since the introduction of synthetic chemical insecticides in the 1940s, they continue to be an effective tool for controlling insects that carry disease pathogens. Unfortunately, insecticides are used indiscriminately and tremendous selective pressure is applied to resist insecticides [9]. Leishmaniasis control can be achieved by interrupting the transmission cycle. The most widely used methods of early detection and treatment of illnesses and infection control vectors and hosts its repository. Although it is often used as a strategy to control the disease leishmaniasis, because of the difficulty of locating terrestrial habitat for mosquito larvae, it is limited. Therefore, vector control further relies on the control of adult mosquitoes, often through the use of chemical insecticides [3]. Sandflies are insects that need to be monitored because they are actively targeted by insecticides [9]. To control sandflies, their populations around the world have been exposed to four main groups of insecticides: Organochlorines, Organophosphates, Carbamates, and Pyrethroids by residual spraying, ultra-low volume spraying, insecticide-treated clothing, and insecticide-treated nets. This exposure is directed either in an attempt to control the vector or as part of the effort to control vectors against other insect vectors; it is inadvertent [4]. Although most species of sandflies are exposed to all major insecticide groups in the world, further evidence suggests that some phlebotomine sandflies may be developing insecticide resistance [10]. Some populations of sandflies are tolerant or resistant to insecticides used in the Middle East, South Asia, and South America [9]. In Montes Claros, Brazil, 29 of 80 (36.3%) Lu longipalpis survived against Deltamethrin 0.05 [11]. 11 of 80 Ph. argentipes (14%) in the Delft Island population of Sri Lanka, had insensitive acetyl cholinesterase and 20 (25%) had high esterase's, that both these results linking resistance to Malathion [4]. Historically, sandflies phlebotomine in India were susceptible to all insecticides before 1976. However, from 1976 to oversee spraying Dichlorodiphenyltrichloroethane (DDT) to control Kalar-azar in Bihar said the problem has provoked resistance among them. During 1979, cases with the highest degree of DDT resistance were reported in Ph. papatasi from northern Bihar, while DDT resistance in Ph. argentipes was first reported from the village of Samastipur region [12]. Until 1978, sandflies were known to be sensitive to insecticides, but resistance to DDT was reported in Ph. papatasi and Ph. argentipes in 1979 and 1990 [2]. The Ph. argentipes in all areas of Muzaffarpur, Vaishali, and Patna in the Bihar state of India and in the village of amahibelha in Sunsari, Nepal are resistant to DDT, respectively 43 and 62% of the population as a result of exposure to DDT died [9]. Such as DDT, Ph. argentipes resistant to Pyrethroids from different regions of India reported [13]. In many vector species, such as sand flies Neotropical phlebotomine, the existence of insecticide resistance has not yet been well studied. Insecticide resistance has not yet been proven in Lu longipalpis but there are some signs of its occurrence in this species [10]. Although in some parts of Brazil and Venezuela development of Lu longipalpis resistance to insecticides in agriculture and mosquito control has been reported. The current action of the IRS in response to human VL cases is geographically discontinuous, temporarily dispersed, and a stable variable, and is unlikely to lead to insecticide resistance due to its detrimental effect on Lu longipalpis accumulation behavior [8].
To provide valid information about these new results, we use reliable data from academic sources such as Google Scholar, Scopus, Web of Science, Springer, Pro-Quest, Wiley Online, Science Direct, Research Gate, PubMed, Sage, and SID we did.
Studies on susceptibility of Visceral leishmaniasis mosquitoes vector to insecticides
Studies on susceptibility of Visceral leishmaniasis mosquito's vector to insecticides and their findings are summarized in Table 2. Kala- azar has been endemic to the Indian continent since 1824 and has caused an epidemic. During the early years of the malaria campaign in India (1953–1958), the incidence of Kala-azar also declined significantly due to the benefit of IRS bail with DDT. Geographical locations Studies on the susceptibility of sandflies to DDT or other insecticides are shown in Figure 3 [2].
| Species | Country | Insecticides | Susceptibility | Source |
|---|---|---|---|---|
| Ph. argentipes | India | DDT | Susceptible | [2] |
| Ph. argentipes | India | DDT,Dieldrin | Susceptible | [14] |
| Ph. argentipes | India | DDT,Dieldrin | Susceptible | [15] |
| Ph. argentipes | India | DDT | Tolerant | [17] |
| Ph. argentipes | India | DDT, Dieldrin | Resistant to DDT and susceptible to Dieldrin | [2] |
| Lu. longipalpis | Brazil | DDT | Studies on the baseline activity of possible DDT-resistance mechanisms | [18] |
| Ph. argentipes | India | DDT | Resistant | [18] |
| Ph. argentipes | India | DDT | Resistant | [19] |
| Ph. argentipes | India | DDT | Resistant | [20] |
| Lu. longipalpis | Brazil | DDT, Chlorpyriphos, Malathion, Propoxur, Deltamethrin | Susceptible to all insecticides studied | [14] |
| Ph. argentipes | India | DDT | Resistant | [21] |
| Ph. argentipes | India | DDT | Susceptible | [22] |
| Ph. argentipes | India | DDT | Susceptible | [23] |
| Lu. longipalpis | Venezuela | DDT=2%, Propoxur=0.01%, Malathion=2%, Fenitrothion=1%, Pirimiphos methyl=1%, Deltamethrin=0.06%,Lambda-cyhalothrin=0.06%, Permethrin=0.2% | Susceptible to all insecticides studied | [16] |
| Ph. kandelakii and Ph. perfiliewi | Iran | DDT | Susceptible | [24] |
| Ph. argentipes | India | DDT, BHC, Malathion, Deltamethrin, Permethrin, Bendiocarb | DDT and BHC=Tolerant, Malathion, Deltamethrin, Permethrin=Resistant Bendiocarb=susceptible | [25] |
| Ph. argentipes | Bangladesh | DDT | Susceptible | [2] |
| Ph. argentipes | Nepal | DDT | Susceptible | [2] |
| Ph. argentipes | Nepal | Malathion, Bendiocarb, | Susceptible to all insecticides | Environmental health |
| Deltamethrin and Lambda-cyhalothrin | Studied | |||
| Ph. argentipes | India | DDT | Resistant and Susceptible | [26] |
| Ph. argentipes | India | DDT and Deltamethrin | DDT=Resistant Deltamethrin=susceptible | [27] |
| Ph. argentipes | Sri Lanka | Malathion | Biochemical evidence of resistance | [28] |
| Lu. longipalpis | Brazil | Malathion, Fenitrothion, λ-cyhalothrin, Permethrin and Deltamethrin | Susceptible | [11] |
| Ph. argentipes | India | DDT and Deltamethrin | DDT=Resistant Deltamethrin=Susceptible | [29] |
| Ph. argentipes | Nepal | DDT and Deltamethrin | DDT=Resistant Deltamethrin=Susceptible | [29] |
| Ph. argentipes | India | DDT, Deltamethrin and Malathion | DDT=Resistant, Deltamethrin and Malathion=Susceptible | [30] |
| Lu. longipalpis | United States | Cypermethrin, Deltamethrin, lambda(λ)-cyhalothrin, Permethrin, chlorpyriphos, Fenitrothion, Malathion, Bendiocarb, Propoxur, DDT | Susceptible | [9] |
| Lu. longipalpis | Brazil | Alpha-cypermethrin | Susceptible | [10] |
| Ph. argentipes | India | DDT | Resistant | [31] |
| Ph. argentipes | India | DDT | Resistant | [32] |
| Ph. argentipes | India | DDT | resistant | [12] |
| Ph. argentipes | India | DDT, Deltamethrin and Malathion | DDT=Resistant Deltamethrin and Malathion=susceptible | [13] |
| Ph. argentipes | Sri Lanka | DDT, Malathion, Propoxur, and Deltamethrin | susceptible | [3] |
| Ph. kandelakii and Ph. perfiliewi | Iran | DDT, Malathion, Propoxur, Lambda-cyhalothrin | Lambda-cyhalothrin=Susceptible Propoxur, Malathion, and DDT= possible resistance | [33] |
Table 2: Status of insecticide susceptibility status in Visceral leishmaniasis sandflies vector in the world.
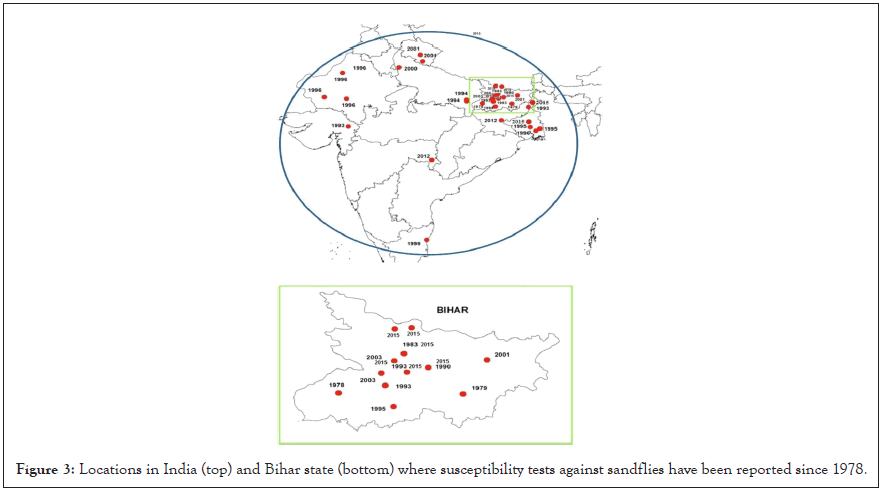
Figure 3: Locations in India (top) and Bihar state (bottom) where susceptibility tests against sandflies have been reported since 1978.
The susceptibility of Ph. argentipes to DDT was also studied in West Bengal in 1959. All sand flies were 95 to 100% susceptible [2]. Kaul published preliminary results on the susceptibility of Ph. argentipes and Ph. papatasi collected from Bihar [14]. In 1979, more accurate results were published by Joshi. This confirmed susceptibility in Ph. argentipes, and resistance in Ph. papatasi [15]. Following indoor residual spraying with DDT, Mukhopadhyay observed rehabilitation of Ph. argentipes in northern Bihar and provided a clue to the possible development of sandfly resistance [16]. Later, Mukhopadhyay first reported the development of tolerance in Ph. argentipes from the Samastipur region of Bihar [17]. In 1991, the National Malaria Eradication Program in India reported a mortality rate of 82-100% in Ph. argentipes collected from the Sahibganj area against DDT test paper [2]. DDT resistance was first observed in Ph. argentipes, a VL vector, from endemic areas of India [18]. After reporting tolerance to DDT in Ph. argentipes, studies on the effect of house spraying DDT on field populations of vector species in Bihar, Uttar Pradesh, and West Bengal in India and Bangladesh and Nepal [2]. In Kaul study, the effect of DDT spraying on field populations of Ph. argentipes in Aishali and Patna districts was investigated. Susceptibility experiments using 4% DDT showed only 15.4% mortality of Ph. argentipes. This study provides field evidence for the development of Ph. argentipes resistance to DDT in Bihar [19]. Joshi and Rai studied the effect of DDT spraying on field populations of Ph. argentipes in Varanasi district, India, and found that Ph. argentipes is susceptible to DDT [20]. In a 1994 study by Oliveira Filho and Melo, the Lu longipalpis field population showed susceptibility to the insecticides tested and showed Lethal Time (LT) values similar to the reference strain [18]. Basak and Tandon [21] and Chandra [22] found resistance in Ph. argentipes from 24 Parganas (West Bengal), India while from the Hoogly region of West Bengal were 100% susceptible. In a study by Mukhopadhyay (1996) in West Bengal, Ph. argentipes was found to be susceptible to DDT [23]. The field population of Lu longipalpis from La Rinconada, Lara State, an endemic focus of Visceral leishmaniasis in Venezuela, was studied for susceptibility to Organochlorines (DDT 2%), Carbamates (Propoxur 0.02%), Organophosphate (Malathion 2%, Fenitrothion l%, and Pirimiphos-methyl l%), and pyrethroids (Deltamethrin 0.06%, lambda-cyhalothrin 0.06%, and Permethrin 0.2%) insecticides and compared with a laboratory reference strain. Lu longipalpis field population showed susceptibility to tested insecticides and showed LT values similar to the reference strain [18]. In a 1994 study by Rassi in Iran, the susceptibility of two vectors of Visceral leishmaniasis, Ph. kandelakii and Ph. perfiliewi were tested for DDT 4% insecticide in Ardabil province (northeast) of Iran. The results showed that both species were completely sensitive to DDT after 60 minutes of exposure with a 100% mortality rate [24]. Amalraj reported tolerance in Ph. argentipes from Pondicherry, southern India against DDT and Malathion but resistance to Permethrin. The study also showed that Bendiocarb, a Carbamates insecticide, may be effective against populations of Ph. argentipes resistant to Organophosphates and pyrethroids [25]. A study by Choudhury in Bangladesh reported Ph. argentipes sandflies susceptible to DDT [2]. In Nepal, according to studies by Anonymous and Project Environmental Health, the same vector in Dhansua was susceptible to DDT [2]. In 2001, varying levels of DDT susceptibility from susceptible to resistant species of Ph. argentipes were reported by Singh [26]. Dhiman also reported resistance to Ph. argentipes from the Vaishali area of Bihar to DDT while being susceptible to Deltamethrin [27]. In Sri Lanka Surendran provided biochemical evidence of increased esterase levels for resistance in Ph. argentipes to Malathion [28] and another study in 2009 by Alexander. On susceptibility to insecticides in two Brazilian populations of Lulongipalpis (Lapinha and Montes Claros) vector of Visceral leishmaniasis. Survival analysis showed that while there was no significant overall difference in the susceptibility of both populations to organophosphates, Lapinha sandflies were significantly more susceptible to Pyrethroids than those from Montes Claros [11]. Dinesh in its study reported 43% mortality with 4% DDT in Ph. argentipes collected from three Bihar regions, and also reported only 62% mortality in one of the villages of the Sunsari region in Nepal [29]. In another study, Singh reported DDT resistance (89.5% mortality) in Ph. argentipes and complete susceptibility to Malathion and Deltamethrin in some parts of India [30]. Denlinger, Lozano-Fuentes in 2015 tested the laboratory susceptibility of Lu longipalpis to 10 insecticides. Despite differences in the killing rates of Carbamates and Organophosphates, Lu longipalpis is most susceptible to Bendiocarb, Propoxur, and Fenitrothion. Conversely, of the 10 insecticides tested, the least susceptible are to DDT [9]. Another study conducted in Brazil by Grasielle Caldas DÁvila Pessoa in 2015 examined the susceptibility of alpha-Cypermethrin in Lu longipalpis. The field assay method showed that the test population at all treated levels was highly susceptible to alpha-Cypermethrin[10]. In 2015, Vijay Kumar tested insecticide susceptibility in Ph. argentipes in two areas in West Bengal, India. Susceptibility status Ph. argentipes to DDT ranged from 40 to 61.54% [31]. Aarti Rama In their study to monitor the susceptibility status for the preparation of resistant sandfly colonies after examining the susceptibility characteristics of Ph. argentipes to DDT for Bihar regions performed DDT susceptibility testing in seven regions of Bihar (India). The Vaishali area was subsequently selected as a "suitable place for collecting resistant sandflies". Here Percentage Mortality Range (PMR) and Corrected Mortality Rate are 41.00-52.73 and 44.83%, respectively, the lowest recorded rates of highly resistant DDT sandflies [32]. In 2017, Aarti Rama conducted a study to evaluate the maximum exposure time that DDT-resistant Ph. argentipes can tolerate the effect of DDT for survival. The mortality rate of laboratory-reared DDT-resistant Ph. argentipes strains exposed to DDT was studied at 60-min intervals, and it was concluded that highly resistant sandflies can withstand this insecticide for up to 420 min. Finally, in 480 minutes of exposure to insecticides, they achieved absolute mortality. Also, LT was observed for female Ph. argentipes more than males, indicating that they are highly resistant to DDT toxicity [12]. The values of LT-50, LT-90, and LT-95 for Ph. argentipes tested were observed with 95% confidence intervals at 280 min, 370 min and 400 min, respectively (Table 3) [12].
| Insecticide exposure time (in min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Observed Parameter | 60 | 120 | 180 | 240 | 300 | 360 | 420 | 480 |
| Control | ||||||||
| No. of sand flies Tested (NT) |
(15 Female, 25 Male); Total=40 | |||||||
| Alive | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Mortality % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Experiment | ||||||||
| No. of sand flies Tested (NT) |
(60 Female, 50 Male); Total=110 | |||||||
| No. of Alive (NA) ± % | 109 ± 99.09% | 106 ± 96.36% | 82 ± 74.54% | 56 ± 50.90% | 32 ± 29.09% | 3 ± 2.72% | 0 ± 0% | 0 ± 0% |
| No. of Senseless (NS) ± % | 1 ± 0.90% | 3 ± 2.72% | 15 ± 13.63% | 22 ± 20% | 13 ± 11.81% | 10 ± 9.09% | 1 ± 0.90% | 0 ± 0% |
| No. of Dead (ND) ± % | 0 ± 0% | 1 ± 0.90% | 13 ± 11.81% | 32 ± 29.09% | 65 ± 59.09% | 97 ± 88.18% | 109 ± 99.09% | 110 ± 100% |
| Observed Mortality=ND/ NT*100 | 0 | 0.90 | 11.81 | 29.09 | 59.09 | 88.18 | 99.09 | 100 |
| LT-50 tested Ph. argentipes against DDT (4%) | 280 min; at CI of 95% | |||||||
| LT-90 tested Ph. argentipes against DDT (4%) | 370 min; at CI of 95% | |||||||
| LT-95 tested Ph. argentipes against DDT (4%) | 400 min; at CI of 95% | |||||||
Table 3: Susceptibility test result for estimating LT-50, LT-90, and LT-95 for resistant Ph. argentipes responded towards the prolonged exposure of DDT at discriminating time intervals of 60 min.
Sardar a study to evaluate the susceptibility of carrier sand fly species (Ph. argentipes) to Deltamethrin (type II Pyrethroids), DDT (Organochlorines), and Malathion (Organophosphate) and to detect polymorphisms in the voltage-gated sodium channel gene (VGC) and They examined their association with susceptibility to pyrethroids type II and DDT in three endemic areas of Kala- azar in West Bengal, India. Polymorphisms were detected in the second domain of segment 6 VGSC gene of pyrethroids and DDT susceptible and tolerant Ph. argentipes by DNA sequencing. It was found that the population of Ph. argentipes in the study area is between 98.02% to 98.80% and 98.81% to 100% susceptible to Deltamethrin and Malathion respectively, but resistant to DDT [13]. In a recent study by Pathirage, patterns of Ph. argentipes insecticide susceptibility were investigated by exploring possible underlying resistance mechanisms. Adult offspring of Ph. argentipes collected from four different regions of Sri Lanka (Mirigama, Pannala, Thalawa, and Mamadala) were exposed to different concentrations of DDT, Malathion, Deltamethrin, and Propoxur using WHO bioassay susceptibility kits [3]. The lowest susceptibility (excluding Deltamethrin) was observed in the Mamadala population, while the highest was observed in the Mirigama population. Increased levels of glutathione S-transferase and esterase activity were observed in sandflies originating from Mirigama, Panala, Talawa, and Medala, respectively [3].
In another study by Rassi 2020 to evaluate susceptibility to insecticides DDT (4%), Malathion (5%), Propoxur (0.1%), and Lambda-cyhalothrin (0.05%) in two vectors of Visceral leishmaniasis in Iran Ph. kandelakii and Ph. perfiliewi collected from the endemic focus of Visceral leishmaniasis region. These species were sensitive to Lambda-cyhalothrin but despite this, they have potential resistance to three other insecticides [33].
Insecticides, in fact under the control of integrated disease management (IDM), play an important role in controlling carrier disturbance to reduce disease burden. For many years, DDT has been used worldwide to control sand flies [9]. Previous reports confirm the decline in VL cases during the 1970s and 1970s, as an advantage of DDT spraying collateral for malaria control programs under the National Malaria Control Program and the National Malaria Eradication Program, launched in 1953 and 1958, respectively and hence populations of Ph. argentipes were also effectively suppressed due to higher levels of DDT susceptibility [25, 32]. Laboratory colonies of insecticide-susceptible sandflies are not very sensitive to DDT. Despite reports of sand flight tolerance and DDT resistance in India, Iran, Nepal, and Turkey, the use of DDT for residual indoor spraying is still permitted. Large doses of DDT are required, which, if not applied properly or at the right time, can create strong pressure for resistance. With years of DDT use and the potential for low tolerability, sandfly populations may develop DDT resistance faster than other insecticides [9]. To date, resistance to DDT has been reported in 2 species of phlebotomine sand fly carriers. For the first time, resistance to Ph. papatasi from northeastern India and more recently from Iran was described. Similarly, DDT resistance was detected in Ph. argentipes (carriers of Visceral leishmaniasis) from endemic areas in India [18]. A review of the literature on sandfly susceptibility in Southeast Asia shows that Ph. argentipes the main vector of VL has developed DDT resistance in previously used areas such as Bihar, Jharkhand, and Maharashtra, and parts of West Bengal. However, Ph. argentipes is resistant to DDT in important endemic areas of kala-azar in India where pyrethroids insecticides have not previously been used. These insecticides should be used as part of a kala-azar vector resistance management strategy [2]. The development of resistance in the VL vector against DDT is a major concern for the kala-azar control program. To have appropriate vector control strategies, regular evaluation of insecticide vulnerability in the kala-azar vector is desirable. The current strategy for controlling Leishmania transmission based on IRS with DDT should be clarified regarding the development of resistance in target species to DDT and other insecticides. DDT is no longer as effective as it was in the 1970s, so it is worrying that the situation may worsen after the development of 100% resistance to DDT in Ph. argentipes [31].
Need to conduct a comprehensive study on the distribution and type of insecticide resistance mechanisms in sandflies, strengthen public health entomology capacity including field resistance data collection system, GIS-based resistance monitoring, and mapping, funding of susceptibility testing kits and supplies, and training of program managers in the field of insecticide resistance management. To control the resistance to insecticides in sand flies and other VL and CL vectors, the use of rotation, mosaic and insecticide mixtures are possible methods [2].
For up-to-date information on vector susceptibility to insecticides, periodic monitoring of insecticides should be performed for susceptibility testing. Irrational long-term use of insecticides may cause tolerance or resistance to the target insects. Studies on the molecular mechanisms of insecticide resistance, such as the identification of molecular markers and biochemical experiments, are also needed. There is a need to establish surveillance in disease- free areas in pre-endemic countries or countries.
Citation: Nasarbadi M, Azarm A, Molaeezadeh M, Shahidi F, Bozorgomid F, Vatandoost H (2021) Monitoring and Mapping of Insecticide Resistance in Vectors of Visceral leishmaniasis in the World. J Infect Dis Preve Med. 9: 251.
Received: 01-Oct-2021 Accepted: 15-Oct-2021 Published: 22-Oct-2021
Copyright: © 2021 Nasarbadi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.