Drug Designing: Open Access
Open Access
ISSN: 2169-0138
ISSN: 2169-0138
Short Communication - (2022)Volume 11, Issue 5
The World Health Organization (WHO) has classified COVID-19 as a pandemic infection due to the global spread of new corona virus infections. Due to this virus infection, millions of people all around the world had to die or endure severe disease. To prepare for a comparable viral pandemic in the future, it will be imperative to discover new therapeutic treatments. Carrageenans have apparently been effective against 12 viruses, including SAR-COV-2. In this investigation, Main Protease (Mpro) and Angiotensin-Converting Enzyme 2 (ACE2) were used as molecular targets for virtual screening of kappa-, lambda-, and iota- carrageenans. When compared to antiviral drugs, the results show that all three carrageenans have substantial binding affinity for ACE2 and Mpro. The binding affinity of iota-carrageenan is greater than that of other compounds. The binding affinity suggests that carrageenans could be utilized to produce potent antiviral drugs.
COVID-19; Carrageenan; Binding affinity; ACE2; Mpro
Carrageenans are linear polysaccharides that have been sulfated in various positions and are composed of alternating (1→3)-β-Dgalactopyranoses and (1→4)-α-D-galactopyranoses (or 3,6-anhydrogalactopyranoses). Due to its emulsifying, stabilizing, thickening, and gelling qualities, carrageenan is frequently employed as an emulsifier, stabilizer, thickener, and gelling ingredient in topical goods, cosmetics, and food preparations. According to their structural features, sulfation patterns, and the presence or absence of 3,6-anhydro bridges in α-linked galactose residues, they are divided into distinct categories. Among them, kappa-, lambda-, and iota- carrageenans are the three most economically used carrageenans. Lambda-carrageenans are formed by galactose units, whereas iota- and kappa- contain equal amounts of galactose and 3,6 anhydrogalactose. Kappa-carrageenan contains one sulfate group, iota-carrageenan contains two per disaccharide at axial positions, and lambda- has close to three equatorial sulfates.1 Red macroalgae such as Chondrous crispus, Kappaphycus alvarezii (Eucheuma cottonii), and E. denticulatum (E. spinosum) are the primary producers of carrageenan. Depending on the species and sometimes even the life stages of the same species, carrageenan structures can change [1].
As a biomolecule, carrageenan has many biological properties, including those of an antioxidant, an antibacterial, an anticoagulant, and an immunomodulator. Carrageenan also possesses a wide range of antiviral qualities. Twelve viruses (SARS-CoV-2, HSV, InfV, hRV, HIV, hCV, hCoVOC43, HPV, TMV, DENV, JEV, and RVFV) were reportedly combated by carrageenans [2].
Nasal sprays, throat sprays and lozenges containing carrageenans are approved common cold prevention options and have been launched in more than 20 countries. These products also have the potential of first line defense to inhibit the infection and transmission of SARS-COV-2 because carrageenans inhibit the viral infection of SARS-COV-2 Wuhan type in a similar way as the Alpha, Beta, Gamma, and Delta variants.3 The most commonly used carrageenan in these products is iota-carrageenan since iotacarrangeenan displayed an at least 10-fold higher efficacy when compared to lambda- and kappa-carrageenan [3].
A severe case of a respiratory illness was reported in Wuhan, Hubei People's Republic of China, during the end of December 2019.4 Due of its genetic similarities to an earlier known coronavirus (SARS-CoV), 2019-nCoV was eventually renamed as SARS-CoV-2 by the International Committee on Taxonomy of Viruses (ICTV). The World Health Organization (WHO) proclaimed Coronavirus Disease-19 (COVID-19), a coronavirus-induced disease, to be a pandemic on March 11, 2020 as a result of its extensive global spread. SARS-COV-2 Omicron B.1.1.529 variant was initially reported to the World Health Organization (WHO) on 24 November 2021 from South Africa.4 Around the world, Coronavirus Disease-19 has been linked to about 128 million confirmed illnesses and more than 2.8 million fatalities as of 30 March 2021 [4].
Although there are already effective COVID-19 vaccines and drugs that can be used to prevent or treat COVID-19, viral variants can provide significant obstacles for these drugs and vaccines. Furthermore, the WHO believes that a future viral pandemic of a similar type is inevitable [5]. Therefore, it is essential to keep working on discovering new drugs that may be used in the early stages of COVID-19 to stop progression.
In this investigation, two enzymes were used as molecular targets against the coronavirus: Mpro from the virus-cell and ACE2 receptor from the host cell. Numerous structural and nonstructural proteins are encoded by the (+) SS RNA virus known as SARS-CoV-2. The Mpro is a non-structural protein that divides two replicase polyproteins into mature proteins needed to enable viral transcription and replication. In this way, we can prevent the spread of the virus by inhibiting Mpro, while tiny molecules that inhibit ACE2's catalytic pocket can alter ACE2's conformation and prevent SARS-CoV-2 from entering host cells by ACE2. In order to prevent virus replication, we chose Mpro, protease as a target, and ACE2 receptor to prevent SARS-CoV-2 entry [6]. Remdesivir and Favipiravir, two widely used antiviral drugs, were utilized in this work as positive controls to compare the binding affinities to the target proteins ACE2 and Mpro.
The input ligand data for carrageenan compounds and antiviral compounds were downloaded as SDF files from Chemical Entities of Biological Interest (ChEBI). The 3D structures of ACE2 and Mpro were obtained from the RCSB protein data bank using PDB IDs 6m0j and 6lu [7]. To assess the binding affinities of various chemicals, the CB-Dock web server (http://cao.labshare.cn/cb-dock2/) was employed6. In this experiment, only the optimal pose of vina scores for each molecule was assessed, as indicated in Table 1. The best poses of the protein-ligand complex pdb file from the CB-Dock online server were uploaded to the ProteinPlus web server (http://protein.plus) for protein-ligand interaction, and PoseView7 was used to evaluate the 2D interaction diagram.
| Compound names | ACE2 | Mpro | ||
|---|---|---|---|---|
| Vina score | Amino acids involved in hydrogen bonds | Vina score | Amino acids involved in hydrogen bonds | |
| Kappa- carrageenan | -8.4 | Tyr196A, Tyr566A, Lys562A | -7.3 | Asn203A, Ser158A, Gln110A |
| lambda- carrageenan | -8.5 | Asp382A, Asn394A, Arg514A, Glu398A, Lys562A, Trp203A | -6.9 | Arg105A, Gln110A, Glu240A, Lys102A, Thr243A |
| iota- carrageenan | -8.5 | Arg514A, Asp206A, Gln102A, Tyr385A | -7.7 | Asp153A, Gln110A, Lys102A, Phe294A, Thr111A, Thr292A, Ser158A |
| Remdesivir | -8.5 | Arg403, Asn33A, Gln96A, Tyr453E, Tyr505E | -7.5 | Arg105A, Gln110A, Thr111A |
| Favipiravir | -6.4 | Gly496E | -5.8 | Asn151A, Asp295A, Gln110A, Thr292A |
Table 1: Binding parameters between ligands and target proteins.
Lambda- and iota-carrageenan had the lowest scores for ACE2 with vina values of -8.5, followed by kappa-carrageenan (-8.4), albeit their scores are extremely close to one another. Remdesivir and Favipiravir, the standard antiviral drugs, showed vina scores of (-8.5) and (-6.4) for ACE2. Iota-carrageenan had the lowest vina score for Mpro, with a value of -7.7, followed by Kappa-carrageenan (-7.3), and lambda-carrageenan (-7.3). (-6.9). Vina scores of (-7.5) and (-5.8) were displayed for Mpro by Remdesivir and Favipiravir (Table 1).
Affinity indicates the extent of the drug's interaction with the receptor. A low vina score denotes a high binding affinity of the protein to the ligand. Drug candidates are picked from ligands that bind firmly to the target protein because the stronger the connections, the more the ligand will influence the physiological function of the target proteins. A high affinity usually results in a reduced dose requirement [8].
All three carrageenans displayed the same binding affinity as Remdesivir for the ACE2 protein target, but they all have better binding affinities than Favipiravir. Both kappa- and iotacarrageenan and Remdesivir had the same binding affinity for Mpro, whereas lambda-carrageenan had a little lower affinity. However, compared to Favipiravir, they all showed a higher binding affinity. Carrageenans demonstrated a higher binding affinity for the ACE2 protein target than Mpro. Iota-carrageenan was the carrageenan with the highest binding affinity for both ACE2 and Mpro.
For structural bioinformatics, pharmaceutical development, and biological research, it is crucial to characterize interactions in protein-ligand complexes. In Table 1 and Figures 1A-1D, it was projected that kappa-carrageenan would bind ACE2 through three H-bonds with Tyr196A, Tyr566A, and Lys562A and Mpro through three H-bonds with Asn203A, Ser158A, and Gln110A. Iota-carrageenan was expected to bind ACE2 through four H-bonding with Arg514A, Asp206A, Gln102A, and Tyr385A and to bind Mpro through seven H-bonding with Asp153A, Gln110A, Lys102A, Phe294A, Thr111A, Thr292A, and Ser158A (Figure 1D). Most high-affinity ligands require the strong hydrogen bonds [9-11].
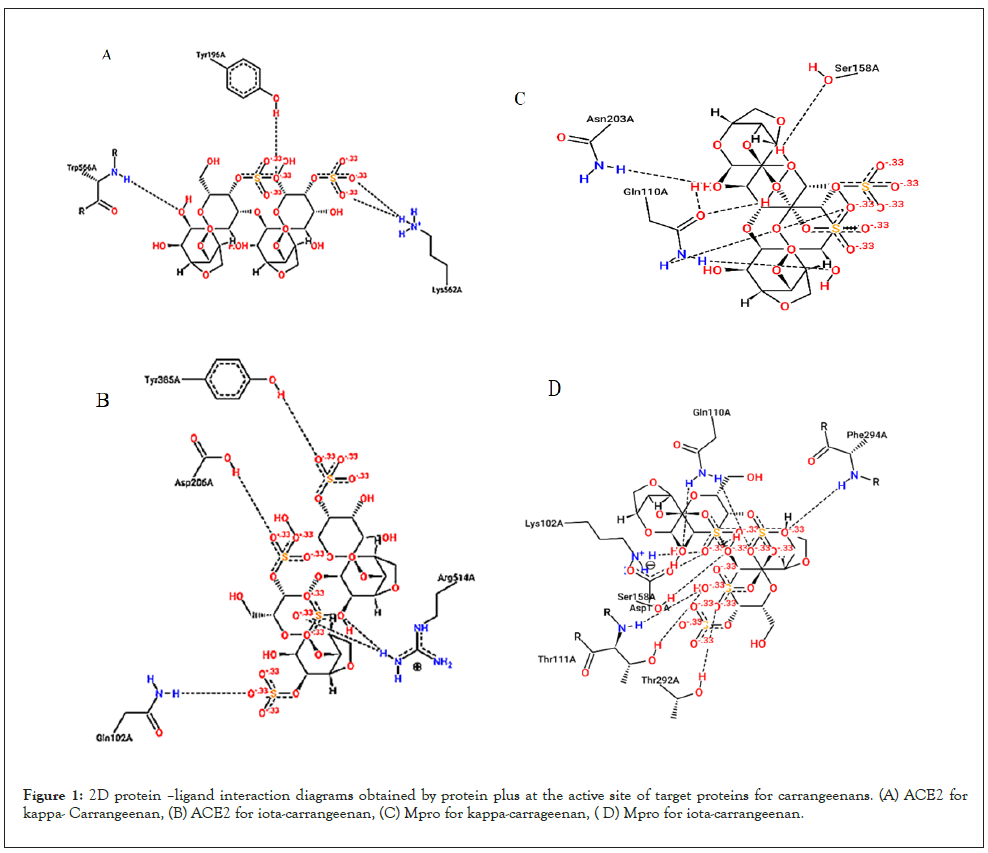
Figure 1: 2D protein –ligand interaction diagrams obtained by protein plus at the active site of target proteins for carrangeenans. (A) ACE2 for kappa- Carrangeenan, (B) ACE2 for iota-carrangeenan, (C) Mpro for kappa-carrageenan, ( D) Mpro for iota-carrangeenan.
It follows that Lambda- and Iota-carrageenan are ligands with high affinity for ACE2 and Mpro. According to the findings, all three carrageenans have the ability to operate as bioactive substances that can protect and treat COVID-19. Iota-carrageenan would be the most effective of these for both treating Carrageenans may be used in future research and development of antiviral agents to combat the Coronavirus. Carrageenans are on the FDA list of substances that are generally recognized as safe for consumption, thus additional ADME (absorption, distribution, metabolism, and excretion) research is not necessary to approve them.
The authors have no conflicts of interest to declare that are relevant to the content of this article.
None
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar].
[Crossref][Google Scholar][PubMed].
[Crossref] [Google Scholar].
Citation: Aung Aung TH (2022) Molecular Docking of Carrageenans for Main Protease (Mpro) and Angiotensin-Converting Enzyme 2 (ACE2) of SARCOV- 2. Drug Des. 11:221.
Received: 19-Oct-2022, Manuscript No. DDO-22-20703; Editor assigned: 21-Oct-2022, Pre QC No. DDO-22-20703 (PQ); Reviewed: 04-Nov-2022, QC No. DDO-22-20703; Revised: 11-Nov-2022, Manuscript No. DDO-22-20703 (R); Published: 18-Nov-2022 , DOI: 10.35248/2169-0138.22.11.221
Copyright: © 2022 Aung TH. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.