Journal of Depression and Anxiety
Open Access
ISSN: 2167-1044
ISSN: 2167-1044
Research - (2020)
Background: The purpose of the study was to present secondary data analyses of an 8-week randomized controlled trial to: 1) Determine the effects of a meditation app on depression and anxiety in adults with sleep disturbance and 2) Explore the potential mediating effect of fatigue and daytime sleepiness on the relationship between use of the app and depression and anxiety.
Methods: Depression, anxiety, fatigue, and daytime sleepiness were assessed at baseline, mid-, and post-intervention in the intervention and waitlist control group. Repeated-measures ANCOVAs assessed intervention effects on depression and anxiety. Mediation models, using the PROCESS macro, were estimated using 5000 bootstrap samples. Per-protocol and intent-to-treat (ITT) analyses were conducted.
Results: Intervention group participants (N=239) had more improvements in depression and anxiety as compared to the control group. Fatigue and daytime sleepiness fully mediated the association between study group and improvements in depression. Fatigue and daytime sleepiness partially meditated the association between study group and improvements in anxiety.
Conclusions: A meditation app may improve depression and anxiety in adults with sleep disturbance with effects being driven by improvements in fatigue and daytime sleepiness. Future studies should consider targeting fatigue and daytime sleepiness to improve mental health in adults with sleep disturbance.
Mental health; Digital interventions; Consumer-based products
Depression and anxiety are leading causes of morbidity in the United States (US) [1]. Each year, approximately 10% US adults suffer from clinically significant depression, and 19% are diagnosed with anxiety disorder [2]. Depression and anxiety negatively impact health, social functioning, quality of life and general well-being [3]. Sleep disturbance is common in those with depression and anxiety [4]. An estimated 10-25% of the general population complain of sleep disturbance [5,6]. Studies have suggested a bidirectional relationship between sleep disturbance and depression and anxiety [7–9], and that each exacerbates and escalate the symptoms of all three conditions [10]. In a systematic review that assessed the bidirectionality of sleep disturbance, depression and anxiety, all studies reviewed (N=10) reported at least one significant relationship between sleep disturbance, depression and anxiety [9]. Additionally, research has shown that individuals with mental health conditions such as depression and anxiety and comorbid sleep disturbance respond less consistently to mental health treatment (e.g., higher rates of attrition, lower rates of remission) and have poorer clinical outcomes (e.g., more severe mental health symptoms, higher risk of suicidal ideation) than those with normal sleep [11]. Although sleep disturbance often has deleterious effects on fatigue [12] and daytime sleepiness [13-15], and depression and anxiety, most sleep research has lacked exploration of daytime symptoms in the treatment of depression or anxiety and/or has not controlled for these symptoms. There is a need to explore treatment strategies to improve depression and anxiety in those who are sleep disturbed.
Depression and anxiety have a range of evidence-based options for intervention. However, barriers exist for many of these treatment options. Both pharmacological and psychological treatment options are typically dependent upon access to mental health care, which remains limited to many [16]. Additionally, both pharmacological and psychological treatment options carry stigma which further limits their use [16]. Because treatment options have a number of barriers, the use of self-management strategies for depression and anxiety such as mindfulness meditation practice is increasing [17]. Further, there is a growing interest in digital tools such as mobile applications (apps) for smartphones, to deliver mindfulness meditation [18]. Mobile apps provide a low cost, practical way to deliver meditation [19] across a broad range of health conditions. There has been a recent surge in the number of individuals utilizing commercially available mindfulness-based smartphone apps [20,21]. However, to date the evidence of the effects of these apps on depression and anxiety is limited [21] and no studies have been conducted in those with sleep disturbance.
In a recent cross-sectional survey of 12,151 subscribers to the Calm meditation app, who had used at least one sleep-related component within the past 90 days, 41% of the sample reported mental health diagnoses (e.g., depression, anxiety, post-traumatic stress disorder) and 76% reported difficulties falling or staying asleep [22]. More than two thirds of participants reported that they started to use the Calm app to improve sleep and over half reported perceived reductions in depression or anxiety [22]. The frequency of using Calm was associated with incremental increases in the likelihood of reporting changes in mental health and sleep. These data, although limited to cross-sectional self-report, suggest that those who are experiencing sleep disturbance may be utilizing mindfulness meditation apps to help them sleep and that they are noticing changes in their mental health at the same time. There is substantial need for experimental studies that can determine the effectiveness of commercially available meditation apps on depression and anxiety, particularly among those who are sleep disturbed [16,23].
Our team recently completed a randomized controlled trial (RCT) examining the effects of using a commercially available mindfulness meditation app (i.e., Calm) on fatigue in those who reported elevated insomnia symptoms [24]. While that study focused specifically on sleep (reported elsewhere) and participants had decreases in fatigue, daytime sleepiness, and cognitive and somatic pre-sleep arousal as a result of the intervention, there were a number of secondary assessments that were designed to examine effects on factors known to be associated with sleep disturbance. The purpose of this paper is to present a secondary data analyses from the parent RCT to determine the effects of Calm on depression and anxiety in adults with elevated insomnia symptoms. We explored the extent to which fatigue and daytime sleepiness, common side effects of sleep disturbance, mediated the relationship between the use of the Calm app and improvements in depression and anxiety symptoms. We hypothesized that those who used Calm would have reductions in depression and anxiety compared to a wait-list control group. We also explored whether reductions in fatigue and daytime sleepiness would mediate the effects of Calm on depression and anxiety.
Study design
This study was a secondary analysis of an eight-week randomized controlled trial testing a mindfulness meditation mobile app on sleep outcomes in adults with sleep disturbance compared to a waitlist control group (trial registration: ClinicalTrials.gov NCT04045275) [24]. A detailed methodology of the parent trial is published elsewhere but briefly described here [24]. The primary outcomes for the current analyses were depression and anxiety, and secondary outcomes were fatigue and daytime sleepiness.
Sample size
Based on the parent RCT, effect sizes were assumed to be moderate (d=0.50). Power analyses indicated 80% power would be achieved with a Neffective of 211. To account for attrition, we aimed to enroll and randomize 250 participants in the parent RCT.
Recruitment and enrollment
All recruitment and enrollment procedures were conducted remotely by a trained research coordinator. Participants were recruited nationally between June and September of 2019, via social media (i.e., Facebook, Instagram), list-servs, and ResearchMatch. org and were asked to complete a 5-minute eligibility screener. Participants were eligible for the parent RCT if they were 1) 18 years or older, 2) willing to download a smartphone app, 3) willing to be randomized, 4) able to read and understand English, 5) considered to have elevated insomnia symptoms (score of ≥10 on the Insomnia Severity Index [25,26], and 6) inexperienced meditators (less than one hour per month for the past six months). Eligible respondents reviewed a video about the study that explained the consent form and completed a brief quiz demonstrating their understanding. After electronic consent was provided, participants completed baseline questionnaires and were randomly allocated to the intervention or wait-list using a list generated from random.org.
Intervention
Participants in the intervention group were asked to download the Calm app and were provided with a free eight-week subscription. The Calm app is a commercially available mindfulness meditation app and provides (but not limited to) general guided meditations, sleep-specific meditations, and Sleep Stories. Participants were asked to meditate for at least 10 minutes daily for eight-weeks and were recommended to start with the “7 Days of Calm” (introduction to meditation), however participants could use the Calm app at their own discretion.
Wait-list control
The wait-list control group was asked to not change their normal routine or use any apps for relaxation, meditation, or sleep for eight weeks. Participants received the same assessments at the same timepoints as the intervention group. At the end of eight weeks and after participants completed their post-intervention surveys, they received a free eight-week subscription to the Calm app.
Measures
For the current study, we were interested in analyzing measures assessing mental health (i.e., anxiety, depression), fatigue, and daytime sleepiness based on our study objectives. All self-reported data was collected using Qualtrics and collected at baseline, four weeks (mid-intervention), and eight weeks (post-intervention) for both groups in the parent trial.
Primary outcomes
Anxiety and Depression was measured using the Depression Anxiety Stress Scales (DASS21) [27] and the Hospital Anxiety and Depression Scale (HADS) [28]. The DASS21 is a self-report measure with three 7-item subscales measuring depression, anxiety, and stress [27]. Respondents use a 4-point Likert-type scale to describe the extent to which statements describing each construct are applicable to their experiences during the past week (0 = Did not apply to me at all, 3 =Applied to me very much or most of the time). Item-level scores are summed within each subscale. The DASS21 has demonstrated adequate internal consistency in the general population (Cronbach’s alphas between 0.91 and 0.84) [29] as well as good convergent and discriminant validity [29,30].
The HADS is comprised of two summated-rating subscales that describe the severity of depression and anxiety symptoms. Each subscale includes 7 ordered-category items that are scored from 0 to 3. Possible subscale scores range from 0-21 with higher scores indicating more severe symptoms. The HADS has demonstrated internal consistency (average α is 0.82 and 0.83 for depression and anxiety subscales, respectively) and validity in both clinical and community samples [31].
Secondary outcomes
Fatigue was measured using the Fatigue Severity Scale (FSS) [29,30]. The FSS is a 9-item scale utilizing a 7-point Likert scale (1 = Strongly disagree, 7 = Strongly agree). Item-level scores are averaged to calculate a scale score, with higher scores indicating greater levels of fatigue. The FSS has high internal consistency (α=.88) and has demonstrated strong convergent validity with physiological and self-report measures of sleep disturbance.
Daytime sleepiness was measured using the Epworth Sleepiness Scale [32-34]. The ESS asks the participants to rate how likely they are to doze off or fall asleep while engaged in eight different activities. Each item is scored from 0 (Would never doze) to 3 (High chance of dozing), such that summated scores range from 0 to 24 with higher scores indicating more daytime sleepiness. The ESS has high internal consistency (α between 0.73 and 0.86) [35] correlates with objective measures of daytime sleepiness and physiological indicators of sleep disturbance, and effectively distinguishes between adults with and without sleep-related diagnoses [34].
Ethics approval
This study was approved by the Institutional Review Board at Arizona State University (Study: 00010050). All participants provided electronic consent via Qualtrics (online survey software) before enrollment.
All analyses were conducted using SPSS 26.0. Descriptive statistics were used to characterize the sample with regard to demographic and health characteristics. Repeated-measures ANCOVAs were used to assess the intervention effects on depression and anxiety. Age, sex, race, and ethnicity were included as covariates in all effects models. Mediation analyses were conducted using the PROCESS macro, version 3.5 [36], in which models predicting post-intervention depression and anxiety were mediated by post-intervention fatigue and daytime sleepiness (Model 4, i.e., simple mediation by a single variable). All mediation models controlled for pre-intervention scores, and included age, sex, and ethnicity and racial minority status as additional covariates. Mediation models were estimated using default bootstrapping procedures (5000 samples). We used Davidson-MacKinnon heteroscedasticity-consistent standard errors and covariance matrices (HC3) to protect against bias due to violations of the assumption of homoscedasticity which could result in standard errors that are biased and inconsistent, such that hypothesis tests based on those standard errors are overly liberal or overly conservative (i.e., inflated risk of Type I error or reduced statistical power) [37,38]. More specifically, HC3 does not impose constraints on the structure of the standard errors, reducing the number of assumptions required for valid inferences, and is appropriate for sample sizes of approximately N=250 or less. To minimize bias related to attrition, both per-protocol and intent-totreat (ITT) analyses were conducted [39]. ITT analyses included data from individuals who discontinued participation in the study prior to protocol completion, but analyses did not include participants who were missing baseline data needed for covariates (i.e., demographic information). Similarly, analyses excluded participants who were missing data describing their baseline levels of fatigue, daytime sleepiness, depression, or anxiety [40]. Given this, sample sizes for individual analyses differ due to completion of relevant baseline data. Sample sizes are provided for each analysis.
Sample characteristics
Two hundred seventy-four participants were enrolled in the study; however, 11 participants were not randomized due to incomplete baseline questionnaires (N=263). See CONSORT diagram (Figure 1).
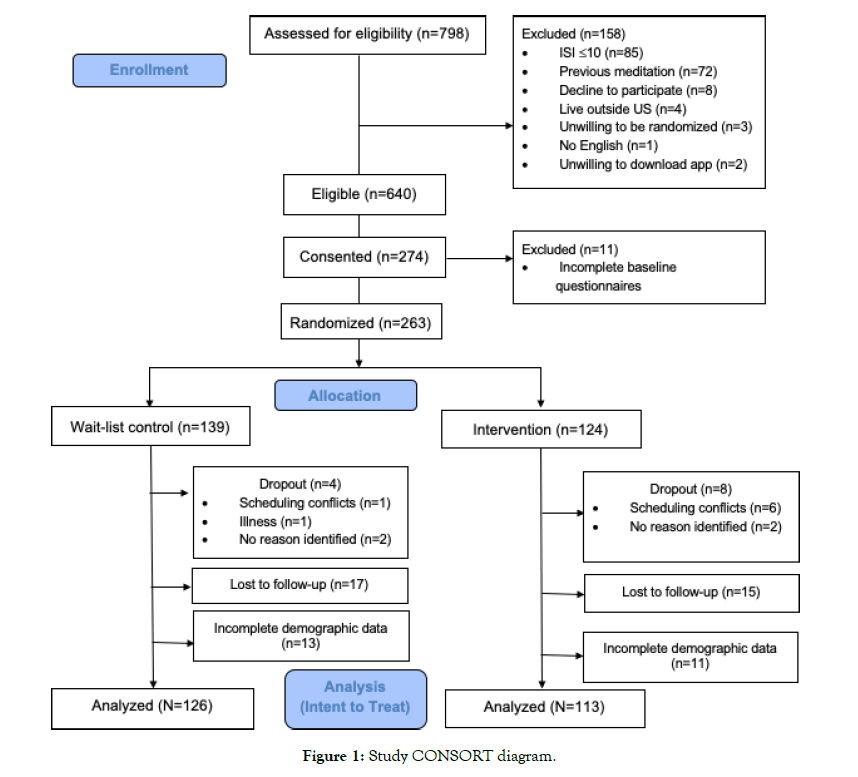
Figure 1: Study CONSORT diagram.
Complete data were available for 239 participants (wait-list control, n=126; Calm n=113; (Table 1). Participants (n=24) who did not complete demographic data were not included in analyses. Perprotocol and ITT analyses yielded similar results; therefore, results presented below reflect the more-conservative ITT analyses. There were no significant differences in attrition rates across study groups (χ2=0.56, p=0.46).
| Variables | Control (n=127) | N Intervention (n=113) | Group Differences | ||
|---|---|---|---|---|---|
| Age, M (SD) | 44.24 | (15.1) | 44.15 | (14.1) | t=-0.05, p=.96 |
| Race, n (%) | χ2=3.68, p=.45 | ||||
| White or European American | 71 | (55.9) | 71 | (62.8) | - |
| Black or African-American | 30 | (23.6) | 19 | (16.8) | - |
| Asian | 17 | (13.4) | 11 | (9.7) | - |
| American Indian or Alaskan Native | 2 | (1.6) | 4 | (3.5) | - |
| Other | 7 | (5.5) | 8 | (7.1) | - |
| Gender Identification, n (%) | χ2<.001, p=.99 | ||||
| Male | 28 | (22.1 | 25 | (22.1) | - |
| Female | 99 | (78.0) | 88 | (77.9) | - |
| Mental health diagnoses, n (%) | |||||
| Depression | 50 | (40.3) | 61 | (56.5) | χ2=6.04, p=.01 |
| Anxiety disorder | 59 | (47.6) | 56 | (51.9) | χ2=0.42, p=.52 |
| PTSD | 18 | (14.5) | 22 | (20.4) | χ2=1.39, p=.24 |
| Baseline mental health symptoms, M (SD) | |||||
| DASS – depression | 7.48 | (6.74) | 9.48 | (6.76) | F=5.11, p=.03 |
| DASS – anxiety | 6.23 | (6.08) | 7.39 | (6.48) | F=1.85, p=.18 |
| HADS – depression | 5.48 | (3.52) | 6.11 | (3.46) | F=1.69, p=.19 |
| HADS – anxiety | 5.94 | (3.52) | 6.52 | (2.95) | F=1.85, p=.18 |
| Baseline Daytime symptoms, M (SD) | |||||
| FSS | 5.79 | (1.72) | 6.15 | (1.81) | F=0.52, p=.47 |
| ESS | 8.34 | (5.00) | 8.33 | (5.18) | F=0.02, p=.90 |
Table 1: Demographic characteristics of study participants.
On average, participants were 44.5 years old (SD = 14.6). Although participants were mostly female (74.6%), the sample was racially diverse (40.6% non-White). Most participants (n=143, 59.8%) reported at least one mental health diagnosis (i.e., depression, anxiety, or post-traumatic stress disorder). There were no significant between-group differences with regard to demographic characteristics. Participants in the intervention group had higher rates of depression diagnoses and higher scores on the DASS21 depression scale at baseline (but not the HADS depression scale).
There were no significant differences in baseline anxiety or daytime symptoms.
Changes in mental health outcomes by group
Mean changes in depression and anxiety scores are presented in Table 2. There were no between-group differences in baseline scores for depression (DASS21: F=2.17, p=.14, d=0.20; HADS: F=0.01, p=.94, d=0.01) or anxiety (DASS21: F=1.23, p=.27, d=0.15; HADS: F=0.09, p=.76, d=0.04). There were a significant group time interaction for DASS21 depression scores and HADS depression and anxiety scores. All interactions indicated that, compared to participants in the waitlist control group, participants using Calm had more improvement in depression and anxiety. However, the group time interaction was not significant in the model of DASS21 anxiety scores.
| Variables | Estimated marginal means | Group × time interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n=124) | Intervention (n=113) | ||||||||
| Pre | Post | Change | Pre | Post | Change | F | p-value | d | |
| DASS21 | |||||||||
| Depression | 7.3 | 6.8 | -0.5 | 9.5 | 7.2 | -2.3 | 4.33 | .01 | 0.20 |
| Anxiety | 6.1 | 5.8 | -0.3 | 7.4 | 5.5 | -1.9 | 1.27 | .28 | 0.15 |
| HADS | |||||||||
| Depression | 5.5 | 5.6 | +0.1 | 6.1 | 5.3 | -0.8 | 5.89 | .004 | 0.32 |
| Anxiety | 5.9 | 6.0 | +0.1 | 6.5 | 5.4 | -1.1 | 8.69 | <.001 | 0.39 |
| FSS | 5.8 | 6.0 | +0.3 | 6.2 | 5.8 | -0.4 | 5.29 | .01 | 0.31 |
| ESS | 8.3 | 8.7 | +0.3 | 8.3 | 7.5 | -0.9 | 6.67 | .001 | 0.35 |
Notes. All models included age, sex, ethnicity, and racial minority status as covariates; coefficients for covariates are available in Supplemental Data 1. Results from analyses of DASS21 Stress subscale scores are available in Supplemental
Data 2. See Huberty et al., 2020 for analyses of the effects of using Calm on changes in fatigue and daytime sleepiness.
DASS21=Depression Anxiety Stress Scale (21-item); ESS=Epsom Sleepiness Scale; FSS=Fatigue Severity Scale; HADS=Hospital Depression and Anxiety Scale.
Table 2: Pre- and post-intervention depression, anxiety, and stress.
Relationships between changes in mental health, fatigue, and daytime sleepiness
Bivariate correlations among the change scores between depression and anxiety, fatigue, and daytime sleepiness are presented in Table 3. There were small (r<.30) to moderate (r<.50) associations between changes in fatigue and daytime sleepiness and changes in depression and anxiety. Changes in fatigue and changes in daytime sleepiness were significantly correlated, but the association was weak. There were significant correlations between changes in the HADS depression and anxiety and DASS21 depression subscale scores, but the relationships across the different measures were weak.
| Variables | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Change in FSS | -- | -- | -- | -- | -- | -- |
| 2. Change in ESS | 0.28*** | -- | -- | -- | -- | -- |
| 3. Change in DASS21 depression | 0.22*** | 0.19** | -- | -- | -- | -- |
| 4. Change in DASS21 anxiety | 0.23*** | 0.17** | 0.68*** | -- | -- | -- |
| 5. Change in HADS depression | 0.35*** | 0.24*** | 0.30*** | 0.15* | -- | -- |
| 6. Change in HADS anxiety | 0.32*** | 0.13* | 0.11 | 0.13* | 0.43*** | -- |
*p<.05, **p<.01, ***p<.001
Note. DASS21=Depression Anxiety Stress Scale (21-item); ESS=Epworth Sleepiness Scale; FSS=Fatigue Severity Scale; HADS=Hospital Depression and Anxiety Scale.
Table 3: Correlations between fatigue, daytime sleepiness, depression, and anxiety.
Given the stronger and more consistent associations between using Calm and changes in HADS scores, mediation models were conducted using the HADS depression and anxiety subscales. In mediation models, study group initially predicted HADS depression scores, however, when controlling for fatigue (Figure 2) and daytime sleepiness (Figure 3) in subsequent models, the direct relationship between group and depression was no longer significant. There were significant indirect effects of fatigue and daytime sleepiness, indicating that improvements in fatigue and daytime sleepiness fully mediated the association between study group and improvements in depression.
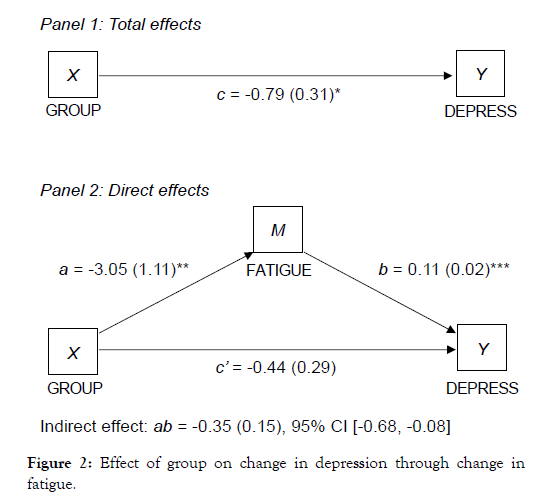
Figure 2: Effect of group on change in depression through change in fatigue.
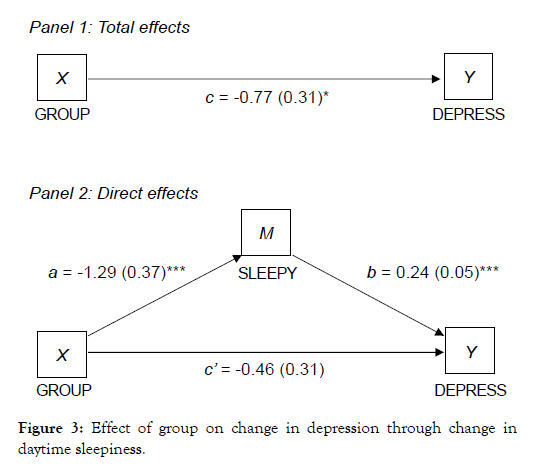
Figure 3: Effect of group on change in depression through change in daytime sleepiness.
Similarly, study group predicted HADS anxiety scores in initial models, but these associations were significantly weaker in subsequent models that controlled for fatigue (Figure 4) and daytime sleepiness (Figure 5). The direct effect of study group on anxiety remained significant, but there were also significant indirect effects indicating that fatigue and daytime sleepiness partially meditated the association between study group and improvements in anxiety.
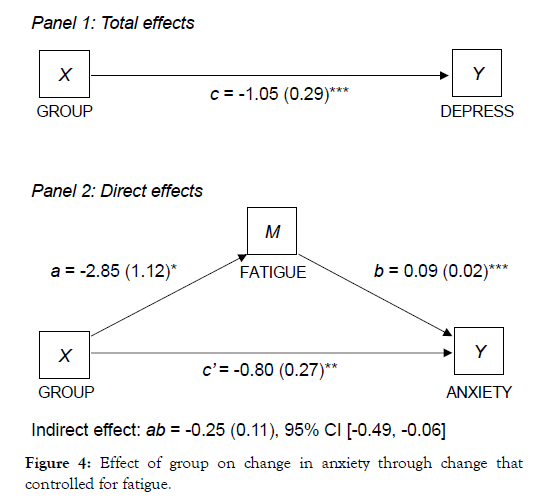
Figure 4: Effect of group on change in anxiety through change that controlled for fatigue.
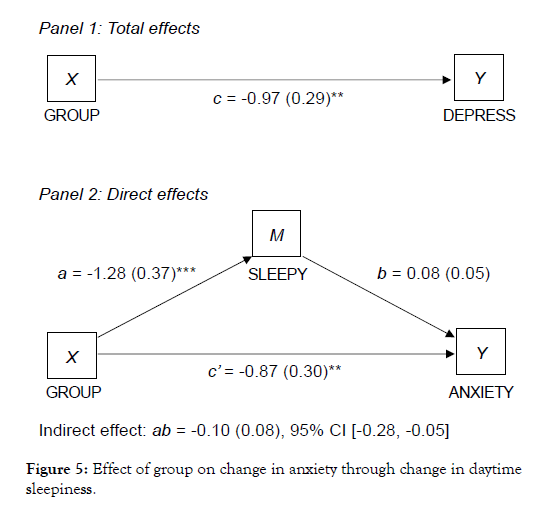
Figure 5: Effect of group on change in anxiety through change in daytime sleepiness.
The findings presented here are secondary data analyses from a previously published parent RCT to determine the effects of using a commercially available mindfulness meditation app (i.e., Calm) on fatigue in those who report elevated insomnia symptoms [24]. The data in the parent RCT suggest that participants had significant decreases in fatigue, daytime sleepiness, and cognitive and somatic pre-sleep arousal. The current study presents findings related to the effects of Calm on depression and anxiety and whether these improvements were explained by improvements in fatigue and daytime sleepiness in adults with elevated insomnia symptoms. To our knowledge the current study is one of the first to assess the effects of using a mobile meditation app to reduce depression and anxiety in those that report elevated insomnia symptoms. Importantly, our findings demonstrate significant reductions in depression and anxiety in those that used Calm for eight weeks as compared to those in the wait-list control group. Changes in depression and anxiety were significantly correlated to changes in fatigue and daytime sleepiness. Finally, changes in fatigue and daytime sleepiness were significant mediators between Calm use and changes in depression and anxiety
Reductions in depression and anxiety
The significant reductions in depression and anxiety in those that used Calm as compared with the wait-list control is not surprising. Mobile apps have become more popular among users to support their mental health [41]. Research suggests that even though many commercial-based apps lack theoretical foundation and/or empirical evidence, some apps have shown promise to be effective in helping individuals address issues related to depression and anxiety. In a study by Flett and Colleagues [17], college-aged adults were randomized to one of three apps [Headspace, Smiling Mind, and Evernote (control)]. After using the intervention apps 10 min/ day for 10 days, participants reported reduced depressive symptoms. Those who used the intervention apps at their own discretion for up to 30 days maintained their reductions in depressive symptoms [17]. A recent systematic review and meta-analysis of standalone apps for mental health reported significant effects of apps compared to controls for overall depression and anxiety [16,42]. Our findings were also consistent with findings from Firth in 2016 and 2017 respectively [16,23] suggesting that apps can be efficacious in reducing depression and anxiety. It is important to note that many studies using mindfulness meditation apps have lacked sham or placebo control groups [43], have been short-term (30 days or less), and have mostly been conducted in the general population [44]. Even though our study used a wait-list control, it was 8-weeks in length and was conducted in those that report elevated insomnia symptoms. More studies are warranted to determine the effects of longer-term interventions using commercial-based mobile apps to improve mental health in clinical populations with stronger control conditions.
Relationships between changes in mental health, fatigue, and daytime sleepiness
It is also not surprising that the changes in depression were significantly correlated with changes in fatigue and daytime sleepiness in our study. Fatigue and daytime sleepiness are important factors in depression [45,46] and are more common in individuals with depression than in those who are not depressed [47]. For many individuals, fatigue is a core symptom of depression [48], and symptoms of depression and fatigue co-occur in a number of chronic conditions [47]. Moreover, several studies have demonstrated that changes in depression over time closely correspond to changes in fatigue [49]. Similarly, daytime sleepiness is also known to be highly associated with depression [47], so much that it has been recommended that a proper clinical evaluation of daytime sleepiness occur in patients with depression [50].
Similarly, we found significant correlations between changes in anxiety and changes in fatigue and daytime sleepiness. Although the associations between depression, fatigue, and daytime sleepiness are well-documented, there is less research that assesses if or how these symptoms are related to anxiety. Findings from both cross-sectional and longitudinal studies have been mixed, with some studies demonstrating relationships between severity of anxiety and severity of fatigue or daytime sleepiness while others fail to detect significant associations [51]. Additionally, most studies that simultaneously assess anxiety and fatigue or daytime sleepiness have been conducted specifically in cancer [52] or other populations with chronic health conditions (e.g., multiple sclerosis [53], rheumatoid arthritis [54], traumatic brain injury [55]) and it is unclear whether these findings extend to other populations.
Role of fatigue and daytime sleepiness as mediators
Changes in fatigue and daytime sleepiness were significant mediators of the relationship between Calm use and changes in depression and anxiety. Specifically, HADS depression scores were fully mediated by changes in fatigue and daytime sleepiness and HADS anxiety scores were only partially mediated by fatigue and daytime sleepiness. Research has shown that sleep difficulties (e.g., insomnia symptoms) may affect the severity and course of mental health problems [11]. In the general population, insomnia and insomnia symptoms often precede depression [56,57] and may trigger depressive episodes [58], whereas research suggests that anxiety tends to appear before or at the same time as insomnia [58]. However, the role of daytime symptoms in onset and maintenance of depression and anxiety has not been well studied. Our findings suggest that fatigue and daytime sleepiness may represent important clinical targets for treating mental health, particularly depression, among individuals experiencing sleep disturbance, and that appbased meditation may be an effective approach for improving these symptoms.
To our knowledge this is one of the first studies to assess the effects of using a mobile meditation app to reduce depression and anxiety in those that report elevated insomnia symptoms. However, there are some limitations that should be noted. First, most of the study participants were women. Women have been reported to use apps for longer periods than men [59] and are more likely than men to use apps for self-care [60]. More research is warranted using a meditation mobile app for depression/anxiety in men who report elevated insomnia symptoms. Second, those who were eligible for the study had to report elevated insomnia symptoms but did not have to have a mental health diagnosis. However, almost half our sample reported some type of mental health diagnosis (e.g., depression, anxiety, post-traumatic stress disorder). This likely underestimates our findings and with a more clinical sample, we may have seen stronger findings and more research is warranted in clinical samples. Third, we had a wait-list control group and the study was not blinded. There are inherent limitations to waitlist control groups and they have been most commonly used as comparators in addition to education and usual care [61,62]. To date app-based meditation interventions have been limited by lack of high-quality control groups. Specifically, few have incorporated active, time- and attention-matched control groups [43]. Future studies warrant active control groups. Finally, assessment of the role of sleep-related symptoms in depression and anxiety could be more nuanced with the inclusion of additional measures of sleep difficulties, insomnia symptoms, and other sleep-related symptoms. In this study we used the Insomnia Severity Index for eligibility not as an outcome measure. Future research warrants the use of additional measures of sleep and related conditions.
Commercial-based mobile meditation apps have become quite popular in the last five years suggesting individuals may be turning to them to help self-manage their health. Depression, anxiety, and sleep disturbance are all common health problems and are all related to each other. Evidence suggests the Calm app may decrease fatigue, daytime sleepiness, and cognitive and somatic pre-sleep arousal. The present study adds to this evidence and suggests that the Calm app may not only help individuals manage their depression and/ or anxiety, with effects being largely driven by improvements in fatigue and daytime sleepiness. This was one of the first studies to explore the extent to which fatigue and daytime sleepiness mediate the effects of app-based-meditation on changes in depression and anxiety in adults with elevated insomnia symptoms.
Dr. Huberty conducts investigator-initiated research with Calm as a partner, but Calm does not financially support her research. Dr. Huberty serves in a consultant role as the Director of Science for Calm. She oversees Calm’s Scientific Advisory Board (SAB) and consults on an as-needed basis to ensure the quality of Calm’s science. She has no specific obligations to the company nor does she own stock in the company or receive financial reward from the sale of the product. Drs. Vranceanu, Larkey, and Irwin also serve on Clam's SAB. They do not financially benefit from the sale of the product nor do they have stock in the company. Mrs. Puzia is a paid employee of Behavioral Research and Analytics, LLC.
Citation: Huberty J, Puzia ME, Green J, Vlisides-Henry RD, Larkey L, Irwin MR, et al. (2020) A Mindfulness Meditation Mobile App Improves Depression and Anxiety in Adults with Sleep Disturbance: A Secondary Analysis of a Randomized Controlled Trial. J Depress Anxiety. 9:374. doi: 10.35248/2167-1044.20.9.374
Received: 25-Sep-2020 Accepted: 21-Oct-2020 Published: 28-Oct-2020
Copyright: © 2020 Huberty J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.