Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Opinion Article - (2015) Volume 4, Issue 1
Product formation from the solvent-free oxidation of aliphatic, cyclic or aromatic thioethers by permanganate supported on copper sulfate pentahydrate has been investigated in detail with respect to the importance of the nature of thioethers, the molar ratio of potassium permanganate absorbed on copper sulfate pentahydrate and reaction activation methods. While microwave irradiation affects appropriately on the fast formation of the sulfones within 3-13 minutes to get the yield more than 74%, ultrasound irradiation activates oxidation of cyclic thioethers mildly to form the corresponding cyclic sulfoxides with the high yields in the range of 83-96%.
Keywords: Potassium permanganate; Oxidation; Thioethers; Microwave irradiation; Ultrasound irradiation
Sulfones and sulfoxides are important commercial compounds, intermediates in organic synthesis and biologically active compounds. [1] They have been mainly produced from the oxidation of thioethers. [2].
In recent decades, with the advancement of science, green chemistry has changed our life style day-to-day [3,4]. Solvent-free reaction plays an important role in the green chemistry due to prevent from solvent contamination, solvent vapour, the elimination of volatile organic solvent, hazards, toxicity and to reduce cost procedure [4]. In solvent-free oxidation reaction, permanganate ion is preferred to be used as “green” oxidation reagent because manganese dioxide formed is easily isolated and treated [3-5]. Moreover, potassium permanganate is a powerful, commercially available and un-expensive oxidation reagent [6]. The permanganate oxidation of thioethers has been investigated under the homogeneous or heterogeneous conditions by using phase-transfer catalysts supported aqueous permanganate solution, [7-9] permanganate complex, [10,11] ground permanganate, [12-14] or inorganic salts/solid supports assisted permanganate ion. [15-19] Furthermore, the efficiency of potassium permanganate on the solvent-free oxidation of thioethers has been shown clearly when it was absorbed on montmorillonite K-10, [20] manganese dioxide, [21,22] Rexyn 101 H, [23] alumina, [24] or inorganic salts [25,26].
Since ultrasound and microwave irradiation were discovered, they have contributed greatly to the green chemistry, specially on the solvent-free reaction aspects [4,27,28]. The presence of ultrasound irradiation has a good effect on the solvent-free oxidation of thioether by potassium permanganate supported on manganese dioxide, [21] besides microwave irradiation has been studied primarily on the solvent-free oxidation of dibenzyl thioether by potassium permanganate supported on copper sulfate pentahydrate [25]. In the continuation of our works on the oxidation by using potassium permanganate absorbed on copper sulfate pentahydrate called xPP/yCSP (x molar amount of potassium permanganate absorbed on y molar amount of copper sulfate pentahydrate), [29] the formation of sulfoxides or sulfones in the solvent-free oxidation of thioethers has inspired us to study under the acceleration of ultrasound and microwave irradiation (Figure 1).
General
Ultrasound irradiations were performed by means of a Branson 1210E-MT ultrasonic bath, operating at 47 kHz. Microwave irradiations were performed by means of a CEM MDS 200 batch microwave oven. GC/MS analyses were performed on a Hewlett Packard 5890 GC 5971A MS apparatus equipped with a J&W DB-5MS capillary column (30 m, 0.25 mm i.d., 0.25 µm film thickness) and a Hewlett Packard 7673A autosampler. NMR spectra were recorded on a Varian Mercury 300 NMR spectrometer.
Oxidation of thioethers into corresponding sulfoxides or sulfones by xPP/yCSP under solvent-free reaction conditions (Method A)
A suitable quantity of finely ground xPP/yCSP was added to a 5-mL round-bottom flask containing the thioether (following the molar ratio as in Table 1) and six glass balls (d=2 mm). The flask was fitted to a shaking machine and shaken at the speed of 280 rpm for a specific period of time (Table 1). Then, the reaction mixture was extracted with 45-50 mL of dichloromethane and filtered through celite layer (2 cm). The extract was removed solvent by rotational evaporation, and then, the remaining crude product was analysed by GC/MS and NMR spectroscopy.
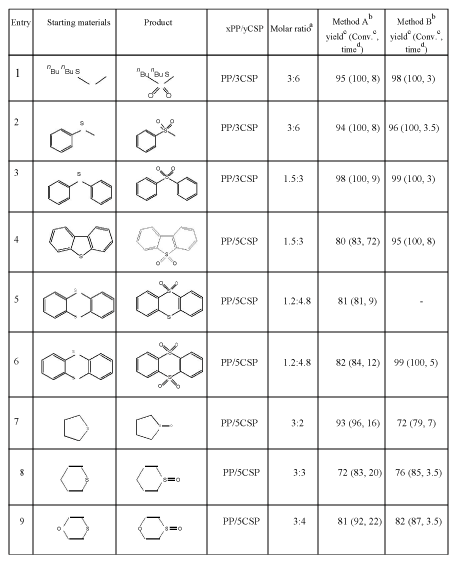 aMolar ratio=the molar ratio of thioether : xPP/yCSP. bMethod A: the reactants were shaken at room temperature. Method B: the reaction was assisted by ultrasound irradiation. cYield and Conv. (conversion yield) were based on GC/MS analysis. dTime=reaction time in hours |
Table 1: Yields of products obtained by the solvent-free xPP/yCSP-promoted oxidation of thioethers by shaking method and ultrasound irradiation
Oxidation of thioethers into corresponding sulfoxides or sulfones by xPP/yCSP under solvent-free reaction conditions assisted by ultrasound irradiation (Method B)
A suitable quantity of finely ground xPP/yCSP was added to a test tube (h=20.0 cm, d=3.0 cm) containing the thioether (following the molar ratio as in Table 1). The test tube was placed into an ultrasound bath where the mixture of reactants was exposed to ultrasound irradiation for a specific period of time (Table 1). Subsequently, the reaction mixture was worked up as described in method A.
Oxidation of thioethers into corresponding sulfones by xPP/ yCSP under solvent-free reaction conditions assisted by microwave irradiation (Method C)
A suitable quantity of finely ground xPP/yCSP was added to a test tube (h=20.0 cm, d=3.0 cm) containing the thioether (following the molar ratio as in Table 2). The test tube was placed into a beaker equipped to adhere test tubes in the CEM (Matthews, North Carolina, USA) oven. For each of the thioethers, an irradiation programme was applied to determine the most efficient reaction conditions, see Table 2. For every experiment, the temperature of the reaction mixture was measured immediately after reaction stop in order to compare with experiments performed under conventional heating. After being cooled, the reaction mixture was worked up as described in method A.
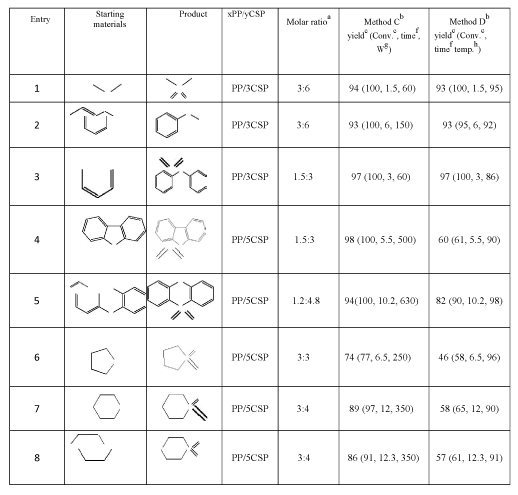 aMolar ratio=the molar ratio of thioether : xPP/yCSP. b Method C: the reaction was assisted by microwave irradiation. Method D: the reaction mixture was heated in an oil bath at appropriate temperature (Experimental). c Yield and Conv. (conversion yield) were based on GC/MS analysis. f Time = reaction time in minutes. g W=power of microwave irradiation in Watts. h Temp =oil bath temperature (Experimental). |
Table 2: Yields of products obtained by the solvent-free xPP/yCSP-promoted oxidation of thioethers by microwave irradiation and conventional heating.
Oxidation of thioethers into corresponding sulfones by xPP/ yCSP under solvent-free reaction conditions assisted by conventional heating (Method D)
A test tube (h=20.0 cm, d=3.0 cm) containing a suitable quantity of finely ground xPP/yCSP and the thioethers (following the molar ratio as in Table 2) were placed in an oil bath heated to the temperature measured at reaction stop of the parallel reaction run under microwave irradiation. The test tube was kept in the oil bath for a period of time corresponding exactly to that found at optimum in Method C. After being cooled, the reaction mixture was worked up as described in Method A.
1H- and 13C-NMR spectroscopic data
The identity and purity of all products reported were ensured by NMR as well as by gas chromatography/mass spectrometry (GC/ MS). Most of products are well known and spectroscopically well characterized already, except the 1H NMR as well as the 13C NMR spectra of thianthrene-5,5-dioxide and thianthrene-5,5,10,10-tetroxide are described below.
Thianthrene-5,5-dioxide (Entry 5, Table 1) white solid, M.p = 165.5-167.5 °C. 1H-NMR (300 MHz) δ (ppm)=8.18-8.24 (m, 2H), 7.62-7.68 (m, 2H), 7.51-7.57 (m, 4H); 13C-NMR (75 MHz) d (ppm) = 135.30, 135.09, 132.04, 128.75, 127.72, 125.44.
Thianthrene-5,5,10,10-tetroxide (Entry 6, Table 1) 1H NMR (300 MHz) d (ppm)=8.31-8.37 (m, 4H), 8.02-8.09 (m, 4H). 13C NMR (75 MHz) d (ppm)=138.26, 134.94, 125.89.
The oxidation of aliphatic thioethers was performed on the basis of work of Shaabani and Lee who used 4 g of potassium permanganate (2 g, 12 mmol) ground with an equal amount of copper sulfate pentahydrate (2 g, 8 mmol) to convert 2 mmol dibutyl thioether into the corresponding sulfone (100%) after five-hour stirring [25]. In our work, the copper sulfate pentahydrate was dissolved in de-ionized water completely, then KMnO4 was added along with a sufficient volume of de-ionized water to obtain a homogeneous solution. The solution was stirred for 10 minutes at 80ºC. Subsequently, water was removed from the solution by rotational evaporation until the weight of the remaining solid mass was equal to the sum of the weights of the original ingredients. The obtained solid mass was ground in a mortar into a fine homogeneous powder.
In order to find the most efficient oxidation agent, a series of experiments was performed, where the molar ratio between KMnO4 and CuSO4.5H2O was varied following the nature of thioether (aliphatic, cyclic or aromatic thioether). The most efficiency of the oxidants investigated, appeared to be PP/3CSP used for the oxidation of aliphatic thioethers and PP/5CSP used for the oxidation of cyclic or aromatic thioethers. In order to achieve the best yield of main product, we also paid attention to the molar ratio between each thioether and xPP/yCSP. The most appropriate molar ratio was also chosen in Table 1.
Altogether eleven thioethers were subjected to oxidation by xPP/ yCSP under solvent-free reaction conditions, using four different methods. In the first series of oxidation reactions where the mixture of reactants was simply shaken together at room temperature (Method A, Table 1), fair to excellent yields were obtained in all cases. The next series of oxidation reactions were performed as described before but under the assistance of ultrasound irradiation (Method B, Table 1). Although the yields of the products in Method B were only slightly improved, the reaction times were shortened considerably. Obviously, ultrasound irradiation has affected well on the heterogeneous reactions (liquid/solid phase) as well as solid/solid phase reactions because cavitation collapse made the surface of solids cleaner, solid articles smaller and mass transfer easily; while above types of reactions often occur incompletely and slowly due to problem with conventional rotational mixing techniques [30-32]. In the Method A and Method B, the oxidation of aliphatic or aromatic thioethers leaded to the formation of sulfones (Entry 1-6, Table 1). However, due to the overlap of unhybridized p orbital of sulfur atom in aromatic thioethers to form a continuous ring of parallel orbitals, electron-donating of aromatic sulfur atom to oxidation reagent happened more difficulty than aliphatic sulfur atom [33-35]. Depending on structure of aromatic thioether, the oxidation did not occur (e.g. the oxidation of thiophene and benzothiophene) or occurred slowly under stronger oxidation conditions (e.g. the oxidation of dibenzothiophene and thianthrene). There was a special product formation in the oxidation of thianthrene (containing two sulfur atoms), where the product from the oxidation of one sulfur atom (thianthrene, 5,5-dioxide) was obtained after ninehour shaking (Entry 5, Method A). Prolongation of the reaction time more than twelve-hour shaking as well as performing the reaction under ultrasound irradiation leaded to the formation of thianthrene, 5,5,10,10-tetraoxide. In the other cases, cyclic thioethers were oxidized into the corresponding cyclic sulfoxides without ring opening (Entry 7-9, Table 1), except the unsuccessful and ring-opening oxidation of 1,4-dithiane. It was explained that the conformation of new-born cyclic sulfoxides (transition products) were not in a plane; therefore, the next oxidation step to form cyclic sulfones took place slowly under the mild conditions.
Under heating activation, transition products could not be existed and reverted to reactants for going on to form stable products in the next step; therefore, the oxidation of all categories of thioethers produced the corresponding sulfones in the short time. It would be of interest to check whether the drastically shortened reaction times could be effected simply by the higher reaction temperatures, so a series of experiments were performed in the presence of microwave irradiation (Method C, Table 2) in comparison with those at the same reaction times and temperatures under conventional heating (Method D, Table 2). The results of experiments (Table 2) clearly demonstrated that a conveniently increased reaction temperature could shorten drastically the formation of sulfone from the oxidation reaction. However, microwave irradiation with “dielectric heating” effects has reached the suitable reaction temperature faster and saved energy much more than conventional heating [36,37].
Comprehensive experimental work has made it possible for us to introduce the xPP/yCSP and emphasize the effects of activation methods on product formation. PP/3CSP and PP/5CSP are also used for fast and solvent-free oxidation of thioethers to get high yield of the products. The assistance of ultrasound or microwave irradiation efficiently shortens the reaction times compared with the shaking or conventional heating methods. Heating activations are suitable for sulfone formation through the oxidation of thioethers.
We thank Danish International Aid Programme (DANIDA) via the Enhancement of Research Capacity (ENRECA) program and Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number [104.01- 2011.40] for financial support.