Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Mini Review - (2022)
Although Intracytoplasmic Sperm Injection (ICSI) was originally considered a promising method for overcoming male factor infertility, its use has been reported to be expanding globally, even in cases of nonmale factor infertility, despite a lack of reliable data demonstrating its benefits. The increasing use of ICSI underscores the importance of improved sperm selection. At the moment, ICSI selection of sperm cells is restricted to motility and morphology under high magnification. Prior to ICSI, it is necessary to generate a pool of spermatozoa that contains the highest quality and competence cells. This increases the likelihood of selecting and injecting superior spermatozoa with superior results. Swim up and density gradient centrifugation are the two current sperm sorting and selection methods used in clinical IVF. Microfluidics is one of the most modern methods for sperm sorting. As compared to conventional sperm sorting methods, Microfluidic Sperm Sorting (MSS) selects sperm that are highly motile and have a low level of DNA fragmentation. We recently published the results of a prospective randomized controlled trial in which we compared the effects of microfluidic sperm selection methods to the traditional swim-up approach in patients with male factor infertility that underwent IVF. The results of this randomized controlled study revealed that the rates of fertilization and the quality of embryos were comparable across the two groups, which was one of the study’s main findings. The study group outperformed the control group in all areas, including live birth and implantation, pregnancy, and clinical pregnancy.
Intracytoplasmic sperm injection; Microfluidic sperm sorting; DNA
Infertility has been recognized as a public health issue worldwide by the World Health Organization (WHO). According to the WHO, approximately 9% of couples worldwide suffer with fertility challenges, with male factors accounting for 50% of the problems [1]. Intracytoplasmic Sperm Injection (ICSI) is a frequently used procedure for In Vitro Fertilization (IVF). Currently, sperm selection for injection is based on subjective morphology assessment, which may not reliably identify the highest-quality sperm. Density gradient centrifugation, sperm washing, and swim-up are the three most common sperm sorting procedures used today. They bypass the natural barriers that sperm would encounter in vivo, and they have been linked to sperm DNA damage that may affect embryo viability [2-4]. To separate sperm cells more closely mimicking natural selection processes than current sperm sorting procedures, microfluidics allows for faster, gentler sorting. Microfluidics is one of the most modern methods for sperm sorting. In comparison to conventional sperm sorting approaches, Microfluidic Sperm Sorting (MSS) chooses highly motile sperm with low levels of Sperm DNA Fragmentation (SDF). Microfluidic sperm sorting appears to result in preparations with a lower percentage of spermatozoa with DNA fragmentation; nevertheless, large cohort findings are still lacking in the published literature. This mini-review will discuss the importance of microfluidic chip technology in the treatment of male infertility, as well as the technology's limitations.
This technology is comprised of disposable microchips and a Charge-Coupled Device (CCD) without a lens. The microchip measures 24 mm × 40 mm in size and is manufactured using a non-lithographic process. From top to bottom, the microchip is made up of three layers: Polymethyl Methacrylate (PMMA), Double-Sided Adhesive (DSA), and a glass coverslip. A microchannel was embedded in the microchip, complete with an input and output. The microchip was mounted on a lens less CCD and illuminated from above in order to obtain shadow photographs of sperm. At a frame rate of one per second, a series of shadow images were acquired using the lens less CCD (Figure 1). In comparison to microscopic imaging, the lens less CCD has a large field of view, allowing it to scan all the sperm in the channel. Real-time recordings of sperm motility were made. Similarly, by turning the device 90 degrees, the complete system may be observed vertically, a significant improvement over conventional microscopic imaging. Thus, the mobility of sperm in both forms may be properly assessed.
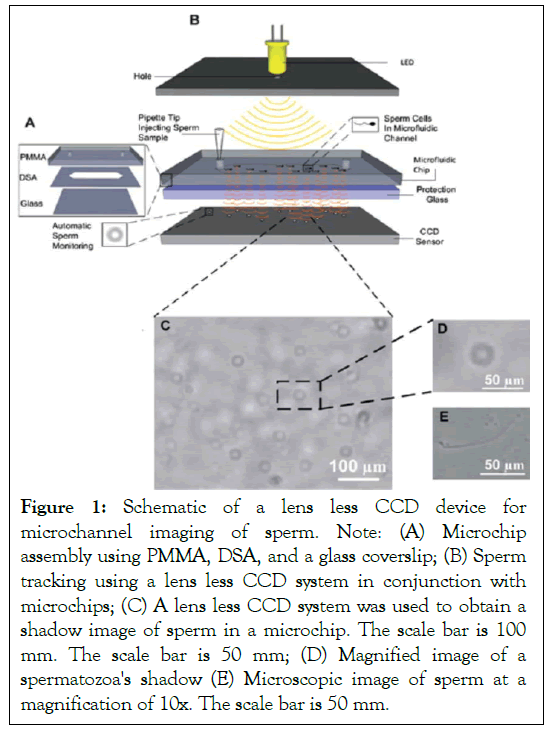
Figure 1: Schematic of a lens less CCD device for microchannel imaging of sperm. Note: (A) Microchip assembly using PMMA, DSA, and a glass coverslip; (B) Sperm tracking using a lens less CCD system in conjunction with microchips; (C) A lens less CCD system was used to obtain a shadow image of sperm in a microchip. The scale bar is 100 mm. The scale bar is 50 mm; (D) Magnified image of a spermatozoa's shadow (E) Microscopic image of sperm at a magnification of 10x. The scale bar is 50 mm.
A Macro-Microfluidic Sperm Sorter (MSS) was developed to simulate natural vaginal sperm separation mechanisms, in which sperm move to the egg via "microchannel" formed by vaginal mucus [5]. Thus, only the most motile and healthy sperm reach the egg and complete fertilization naturally. A porous filter sperm sorting microchip with an embedded polycarbonate nucleoporin trace-etched membrane was designed to duplicate this technology (Figure 2). For optimal sperm sorting, different pore sizes (3, 5, and 8 m) were evaluated. After injecting 560 L of unprocessed sperm into the chip, it was incubated at 37°C for 30 minutes before sperm was recovered from the outlet following filtration. The sorted sperm contained many fewer Reactive Oxygen Species (ROS) and DNA fragmentation than conventionally sorted sperm [5]. Thus, utilizing a disposable microchip, the most motile and functioning sperm were preferentially filtered through this filtering process, and the dead or less motile sperm were kept by the filter membrane. The described microchip is simple to use, extremely effective, and capable of standardizing sperm sorting in clinical settings without centrifugation, thereby reducing sorting discrepancies between operators.
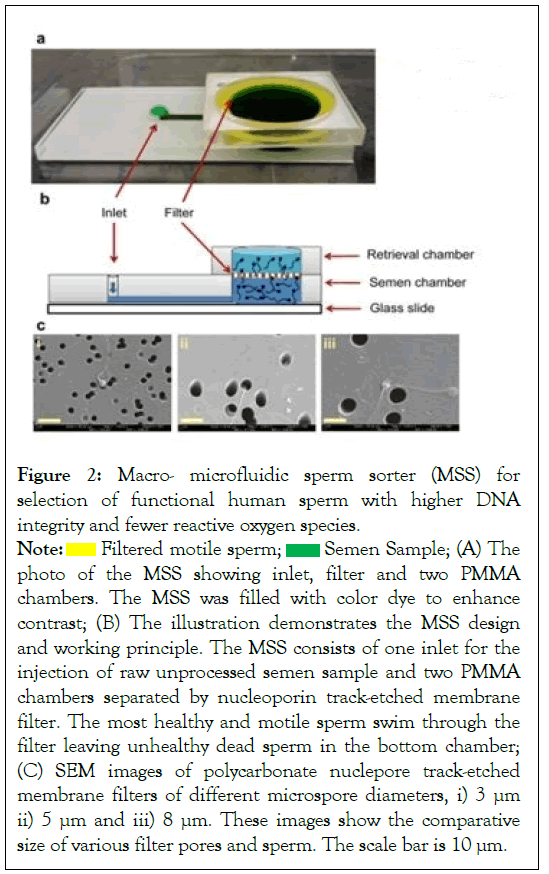
Figure 2: Macro- microfluidic sperm sorter (MSS) for
selection of functional human sperm with higher DNA
integrity and fewer reactive oxygen species.
Note:  Filtered motile sperm;
Filtered motile sperm;  Semen Sample; (A) The
photo of the MSS showing inlet, filter and two PMMA
chambers. The MSS was filled with color dye to enhance
contrast; (B) The illustration demonstrates the MSS design
and working principle. The MSS consists of one inlet for the
injection of raw unprocessed semen sample and two PMMA
chambers separated by nucleoporin track-etched membrane
filter. The most healthy and motile sperm swim through the
filter leaving unhealthy dead sperm in the bottom chamber;
(C) SEM images of polycarbonate nuclepore track-etched
membrane filters of different microspore diameters, i) 3 μm
ii) 5 μm and iii) 8 μm. These images show the comparative
size of various filter pores and sperm. The scale bar is 10 μm.
Semen Sample; (A) The
photo of the MSS showing inlet, filter and two PMMA
chambers. The MSS was filled with color dye to enhance
contrast; (B) The illustration demonstrates the MSS design
and working principle. The MSS consists of one inlet for the
injection of raw unprocessed semen sample and two PMMA
chambers separated by nucleoporin track-etched membrane
filter. The most healthy and motile sperm swim through the
filter leaving unhealthy dead sperm in the bottom chamber;
(C) SEM images of polycarbonate nuclepore track-etched
membrane filters of different microspore diameters, i) 3 μm
ii) 5 μm and iii) 8 μm. These images show the comparative
size of various filter pores and sperm. The scale bar is 10 μm.
While the male factor is known to be the only cause of infertility in around 20% of infertile couples, it is also known to be a significant role in approximately 30%-40% of female infertile couples [6,7]. Semen analysis is a critical component of evaluating male infertility. Motility, morphology, viability, DNA integrity, apoptosis, and maturation of the sperm are all significant determinants of Assisted Reproductive Technologies (ART) success. Recently, it has been discovered that the integrity of sperm DNA is critical for normal fertilization and embryo development. As a result, improved sperm selection procedures are employed to identify higher-quality and healthier sperm for use in ICSI treatment. Although it has been demonstrated that the great majority of these new sperm selection methods discussed previously may select for sperms with a higher DNA integrity and a lower DNA fragmentation rate. Published a prospective randomized controlled study comparing the effects of microfluidic sperm selection methods to the traditional swimup approach in patients with male factor infertility that underwent IVF. The results of this randomized controlled trial demonstrated that the rates of fertilization and the quality of the embryos, which were among the study's primary findings, were similar between the two groups. The study group had a higher live birth rate, as well as a higher rate of implantation, pregnancy, and clinical pregnancy [8]. This research is remarkable in that it discovered statistically significant variations in the rates of implantation, pregnancy, clinical pregnancy, and live birth. Although the two groups had equivalent numbers of grade 1 and 2 embryos, the control group had a larger number of grade 3 embryos. This might indicate that the Fertile Chip was used to select higher-quality sperm or those other factors affecting embryo grade are not reflected in the morphology of sperm. One study in the current literature compared the effects of microfluidic chips for sperm selection in ICSI cycles to those of gradient-density centrifugation in male infertility patients. There were no statistically significant differences in Clinical Pregnancy Rate (CPR) and continued pregnancy rate between the groups; however they were significantly greater in the microfluidic sperm sorting chip group when used for male infertility [9]. The pregnancy rate increased more significantly among couples with a total motile sperm count of between 1 and 5 million (p 0.01). However, this was a retrospective study in which the spermatozoa of the study group exhibited abnormal morphology and the groups were not homogeneous. In addition to being simple to use and economical, the microfluidic sperm sorting chip is also chemical-free, mechanical-free, and perturbation-free, and it eliminates the need for a centrifuge. At an appropriate time point, only the most motile and functional spermatozoa with the proper structure, high DNA integrity, and a low ROS level may be selectively passed via the microchannel of a microfluidic sperm sorting device, leaving behind the less motile or immotile spermatozoa [5]. The increase or decrease in DNA fragmentation levels observed in gradient technique applications, as well as the conflicting results achieved in previous studies, may be due to initial cellular DNA fragmentation rates or centrifugation. Conducted a 2018 study in which they compared traditional sorting procedures to those using microfluidic chips. According to the study's findings, DNA fragmentation rates were much lower when utilizing the microchip approach than when using the gradient method [10]. For the evaluation of a new strategy for choosing spermatozoa with good chromatin integrity, Parella et al. used density gradient selection and microfluidic sperm sorting. As revealed in this study, microfluidic selection produced spermatozoa with high genomic integrity and increased the possibility of developing a conceptus with a euploid chromosome sequence [11]. Guler et al. has published a prospective randomized controlled research comparing the effects of density gradient centrifugation and microfluidic chip sperm preparation methods on embryo development in patient populations with astheno-teratozoospermia. While the density gradient group had a greater sperm concentration, the microfluidic chip group hadmuch higher motility (progressive and total). According to the result of the research, there were no significant variations in the fertilization rates or the proportions of grade 1 and grade 2 embryos on the third day. Additionally, whereas the proportions of poor, fair, and good blastocysts on day 5 did not differ significantly, the microfluidic chip group had a significantly greater proportion of outstanding blastocysts (indicating high-quality embryos). In individuals with asthenoteratozoospermia, the microfluidic chip sperm preparation produced sperm with increased motility and higher quality blastocysts on day 5 [12].
The Female Reproductive Tract (FRT) is a complex system that requires precise sperm selection. In assisted reproduction techniques, sperm are typically selected based on their density or motility, which are traits that do not represent their ability to fertilize an oocyte and, as a result, may result in the oocyte not fertilizing successfully. The investigation of surrogate markers of fertilization potential, as well as the simulation of natural sperm selection processes, has made significant strides. Although there are several methods of sperm isolation, some of them, such as hyaluronic acid-based selection and microfluidic isolation based on sperm tactic responses, use only one or two parameters and are not comparable to the multistep sperm selection processes that occur naturally within the FRT. Fertilization-competent sperm require a complex set of molecules, including zona pellucida-binding proteins and ion channel proteins, in order to advance through the FRT to fertilize the egg, which is accomplished through the FRT. For the best artificial sperm selection method, a tool that takes into account both the molecular signature of sperm that have a good chance of fertilizing, as well as how they respond to outside stimuli, will be needed.
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
Citation: Ayd?n S, Deniz ME(2022) Microfluidic Chips for Male Infertility Patients. Andrology. S2:002.
Received: 20-Apr-2022, Manuscript No. ANO-22-17001; Editor assigned: 25-Apr-2022, Pre QC No. ANO-22-17001 (PQ); Reviewed: 17-May-2022, QC No. ANO-22-17001; Revised: 24-May-2022, Manuscript No. ANO-22-17001 (R); Published: 31-May-2022 , DOI: 10.35248/2167-0250.22.S2.002
Copyright: © 2022 Ayd?n ,S et a.l This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.