Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Short Communication - (2025)Volume 16, Issue 6
Menopause, whether natural or induced, can lead to metabolic imbalances, altered vascular reactivity and cardiac dysfunction. Estrogen Replacement Therapy (ERT) has been explored as a potential protective measure for cardiovascular health during menopause, but conflicting clinical results exist regarding its effects. Studies suggest that early initiation of ERT may be more beneficial, with research like KEEPS and ELITE supporting the "Timing hypothesis." Ovariectomy, a model for menopause, has been shown to cause metabolic and vascular changes, with early ERT potentially preventing cardiac and vascular deterioration. Overall, the timing of ERT initiation is crucial and these findings emphasize the importance of considering the temporal aspects of the menopausal transition in cardiovascular health studies.
Menopause; Estrogen replacement therapy; Cardiac function; Aging; Metabolism
The natural or induced menopause can cause a metabolic imbalance, alterations in vascular reactivity and cardiac dysfunction [1-3]. The association between menopause and increased incidence of Cardiovascular Disease (CVD) has led to the hypothesis that Estrogen Replacement Therapy (ERT) may protect the cardiovascular system. However, Clinical studies on the effects of ERT have yielded controversial results. Some studies have shown adverse effects on the cardiovascular system [4], while others have confirmed its cardioprotective effects [5]. The differences in results were attributed to the age of the women included in the studies. Aging is closely related to menopause and leads to various changes, including inflammation and cardiac dysfunction [6]. Clinical trials have suggested that ERT has cardioprotective effects when initiated soon after the onset of menopause. Conversely, ERT may have the opposite effect when initiated 5 or 10 years later. To test this theory, known as the “Timing hypothesis”, two trials were conducted: The Kronos Early Estrogen Prevention Study (KEEPS) and the Early versus Late Intervention Trial (ELITE). The KEEPS trial did not find evidence that ERT slows the progression of atherosclerosis [7], while the ELITE trial showed that only early ERT can prevent atherosclerosis [8].
We have recently reported that ovariectomy causes metabolic alterations, including increased body weight, dyslipidemia and insulin resistance. Additionally, it promotes vascular dysfunction by increasing the contractile response to Ang II [9,10]. The dysregulated activity of the Renin-Angiotensin System (RAS) can result in volume overload and vasoconstriction, leading to elevated left ventricular diastolic filling pressures [11]. This, in turn, increases preload, resulting in greater cardiac contractility through elevation of Heart Rate (HR), Stroke Volume (SV) and Cardiac Output (CO), via the Frank-Starling law. The sustained increase in preload causes hypertrophy in the myocardium, leading to an increase in Left Ventricular (LV) mass (Figure 1).
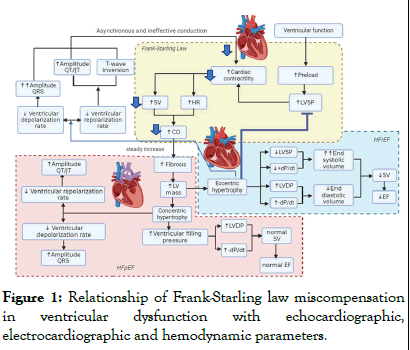
Figure 1: Relationship of Frank-Starling law miscompensation in ventricular dysfunction with echocardiographic, electrocardiographic and hemodynamic parameters.
Ventricular function follows the Frank-Starling law: Increased preload leads to increased Left Ventricular Systolic Pressure (LVSP), which increases cardiac contractility by increasing Heart Rate (HR), Stroke Volume (SV) and Cardiac Output (CO). In Cardiovascular Disease (CVD), sustained elevation of preload can induce myocardial hypertrophy, increasing Left Ventricular (LV) mass. Eccentric hypertrophy involves ventricular dilatation, which enlarges the ventricles and decreases LVSP and +dP/dt, while increasing LV Diastolic Pressure (LVDP) and -dP/dt, despite reduced ventricular volume at end-diastole. Contractile failure causes incomplete systolic blood ejection, increasing endsystolic volume and decreasing ejection volume, leading to Heart Failure with reduced Ejection Fraction (HFrEF) and diastolic heart failure. Ventricular remodeling can lead to slower depolarization and repolarization, reflected in QRS prolongation and QT amplitude with T-wave inversion. In concentric hypertrophy, the reduction in cavity diameter increases ventricular filling pressure, maintaining normal VE and EF, but also decreases depolarization velocity, indicating Heart Failure with preserved Ejection Fraction (HFpEF).
Metabolic and vascular alterations can cause an imbalance in the heart's energy demand, leading to ischemia and myocardial infarctions. This can result in electrocardiographic alterations such as ST-segment elevation and QRS complex fragmentation, which contribute to cardiac remodeling [9]. As part of the adaptive process of cardiac tissue to these new conditions, fibrosis increases, causing cardiomyocyte hypertrophy and thinning of cardiac fibers. At this point, males and females tend to develop different types of cardiac remodeling. Male patients often present with eccentric hypertrophy, which is characterized by ventricular dilation resulting in increased volume of the cardiac chambers. This leads to a decrease in Left Ventricle Systolic Pressure (LVSP) and the maximal rate of rise of left ventricle pressure (+dP/dt), while Left Ventricle Diastolic Pressure (LVDP) and the minimal rate of decrease of left ventricle pressure (-dP/dt) increase. However, the ventricular volume at the end of diastole is lower. Due to contractile deterioration, the myocardium is unable to expel all of the blood during systole, resulting in a greater end-systolic volume and a decrease in the ejected volume. This decrease is reflected in a decreased Ejection Fraction (EF), which causes Heart Failure with reduced Ejection Fraction (HFrEF). Ventricular remodeling can cause slower depolarization and repolarization, which may be reflected in QRS prolongation and QT amplitude with T wave inversion. Females, on the other hand, tend to develop concentric hypertrophy, which is characterized by a reduction in the internal diameter of the cavities and an increase in ventricular filling pressures. Due to the reduction of the chambers, the LVDP increases and the -dP/dt decreases, maintaining a normal SV and a preserved EF, resulting in Heart Failure with preserved Ejection Fraction (HFpEF). Additionally, there is a decrease in the speed of depolarization and repolarization, although less frequently than in HFrEF.
Our previous studies found that early ERT can prevent the deterioration of cardiac and vascular function, as well as metabolic imbalances in lipids and glucose Lopez et al. showed that early but not late ERT prevents an increase in mitochondrial hydrogen peroxide levels, oxidative damage to lipids and proteins and a decrease in glutathione peroxidase and catalase activity. This effect is attributed to a change in estrogen receptor expression, with ovariectomy increasing the ERα/β ratio, which early ERT prevents [12]. However, some morphological alterations indicative of ischemic processes were also reported even with the early ERT administration. This suggests that bilateral ovariectomy leads to a decrease in several peptides and hormones beyond estrogens, so ERT may not be sufficient to prevent all the changes associated with ovariectomy. These investigations have shown that certain physiological and biochemical biomarkers may vary depending on the time elapsed after the loss of sex hormones and the start of the ERT. Thus, changes in cardiovascular health are dependent on the menopausal transition beyond aging.
Menopause causes time-dependent metabolic changes leading to vascular dysfunction by increasing RAS activity and the onset of HFpEF. ERT can prevent cardiovascular dysfunction when initiated early after hormone loss, highlighting the importance of studying this process from a temporal perspective.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hernandez DR, Martinez DL, Monroy JF (2025) Menopause Transition: Early vs. Late Estrogen Replacement Therapy. J Clin Exp Cardiolog. 16:953.
Received: 07-Mar-2024, Manuscript No. JCEC-24-30046; Editor assigned: 09-Mar-2025, Pre QC No. JCEC-24-30046 (PQ); Reviewed: 22-Mar-2025, QC No. JCEC-24-30046; Revised: 01-Jun-2025, Manuscript No. JCEC-24-30046 (R); Published: 08-Jun-2025 , DOI: 10.35248/2155-9880.25.16.953
Copyright: © 2025 Hernandez DR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.