Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2023)Volume 12, Issue 4
Purpose: To determine correlation between lifestyle risk factors and sperm quality.
Methods: Patients (n=133) who consented for the study completed a lifestyle questionnaire. An aliquot of sperm was frozen at three different time points. Preparation methods for 30 semen analysis were compared: ZyMōt Sperm Separation Device (DxNow), Isolate gradient (Irvine), SpermGrad gradient (Vitrolife), and each gradient was followed by swim-up (SU), Isolate+SU and Spermgrad+SU. All samples were analyzed using the Sperm DNA Fragmentation Assay (acridine orange/flow cytometry SDFA™). Analysis included DNA Fragmentation Index (DFI), Oxidative Stress Adducts (OSA) and High DNA Stainability (HDS). Statistical analysis was performed using JMP (SAS 2018) and P<0.05 was considered statistically significant.
Results: The neat DFI was not correlated with age, morphology, or oligospermia (<20 million/mL). Men that consumed alcohol daily trended towards a higher DFI than those that drank multiple times per week and significantly higher than those who never drink (p=0.0608 and p=0.0290, respectively), but interestingly not those who drank rarely. DFI was also positively correlated with OSA and HDS in the neat and processed sample (INSEM). The DFI of the INSEM sperm sample was positively correlated with age, poor morphology, and oligospermia (p=0.0208, p<0.0001, p=0.0006, respectively). There was no correlation with BMI or smoking status for neat or processed sperm health. The separation device effectively improved the DFI, OSA, and HDS compared to other methods
Conclusion: Lifestyle factors and preparation method is correlated with sperm quality.
Sperm DNA fragmentation; Male infertility; DNA stainability; Spermiogenesis
The role of the male partner and sperm health in infertility, pregnancy loss, and the health of the offspring has been mostly unknown and presumed insignificant. The primary focus on improving In-vitro Fertilization (IVF) outcomes has been on oocyte quality, embryo culture, pre-implantation genetic diagnosis, embryo selection tools such as time-lapse or artificial intelligence, and optimizing the timing of transfer for uterine receptivity. Infertility testing for the male partner has historically been a screening semen analysis to check for sperm concentration, motility, and morphology primarily. Additional testing for the male partner typically is only performed if the semen analysis is severely abnormal, such as complete azoospermia. The clinical utility and availability of Sperm DNA Fragmentation (SDF) testing has become more widely recognized in recent years. Focus on the incidence and implications of SDF through testing has led to increased attention to the health of the sperm. However, there is still much unknown. SDF has been shown to be more prevalent in men with certain lifestyle factors, such as smoking, but it is also prevalent in men with advanced paternal age, varicoceles and oligospermia [1-7]. However, men with completely normal semen profiles can also have high SDF with no other factors [8,9]. SDF testing currently is not routinely run as part of the screening infertility testing but is generally used for specific clinical scenarios such as repeat pregnancy loss and cycles [10]. Unfortunately, these scenarios often follow adverse painful and emotionally difficult situations. Performing SDF assays, which are often covered by insurance, are affordable, and can be done from the patient’s home, could potentially save couples from adverse outcomes, emotional distress, and money spent on treatments with low chances of success. Elevated SDF has been shown to be associated with poor outcomes with natural conception and IUI. Not only does the chance of success decrease drastically, but the risk of pregnancy loss also increases. By choosing to move forward with IVF, the couple can save money, time, and the stress and heartache of unsuccessful treatments or miscarriage. SDF has been shown to decrease the success of IVF treatments as well. SDF is correlated with poor fertilization, poor embryo development, poor embryo quality, slower embryo morphokinetics, poor implantation rates, and increased pregnancy loss [11-18]. The physician, based on the SDF results, may decide to look for possible causes and treat the male partner before treating the couple. However, due to the complexity of SDF, the cause is often unknown. Instead, the treatment plan may change, increasing the couples’ chance for a successful outcome.
There are different assays and methods available that measure SDF. The methodology used in this study, Sperm DNA Fragmentation Assay (SDFA™, the acridine orange and flow cytometry as described in the Sperm Chromatin Structure Assay (SCSA©) method, ReproSource), also is comprised of other useful components with clinical significance. These are Oxidative Stress Adducts (OSA™) and High DNA Stainability (HDS). The OSA is measured by a quantification of peroxidation of the lipids in the sperm membrane caused by oxidative stress. Some levels of oxidative stress are needed for normal sperm function, such as in the compaction of histones to protamine. However, abnormal levels of oxidative stress cannot only alter the sperm membrane and morphology, it can cause functional issues with the acrosome, and it can also disrupt the compaction of histones resulting in a high level of histone retention [19]. HDS is a measurement of the percentage of cells with high levels of protamines [20]. This may be indicative of immature sperm that have not completed the final stages of spermiogenesis and thus also may be aneuploid or have altered sperm function. Studies have found correlations between histone retention, oxidative stress, and men with infertility. The components of the SDFA measure the overall health of the sperm and are a powerful predictive tool for fertility treatment outcomes and should be routinely used as part of the diagnosis before the onset of treatment.
Reactive Oxygen Species (ROS) have been a significant focus when looking for the cause of SDF both in-vivo and in-vitro. The presence of ROS, both in the laboratory, induced by or in the body, and generally in the epididymis, causes DNA damage as well as functional damage to the sperm [21-24]. ROS, such as oxygen, hydrogen peroxide, hydroxyl radicals, hypochlorous acid and nitric oxide, are highly reactive molecules with short half-lives [1,2,25,26]. One of the greatest sources of ROS, particularly in men with infertility, is the immature sperm that fail to undergo apoptosis and give off high levels of ROS [25-27]. Other endogenous sources of ROS are mitochondrial respiration and the by-products of different enzymatic systems such as Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase [28]. ROS can indirectly cause sperm damage by activating sperm endonucleases and caspases, which are highly efficient at initiating sperm DNA damage [1-3,28].
Many of the ROS that cause SDF are from exogenous sources such as chemotherapy, radiation, ultraviolet lights, cigarette smoking, daily alcohol consumption, drugs such as acetaminophen or antidepressants, air pollution, chemicals in products that we consume, and many sources yet to be determined [1-3]. Studies have shown that the cytotoxic effects of cancer treatments will affect the level of SDF before and after treatment and can lead to decreased fertility and even complete azoospermia [29-31]. Certain factors such as age, smoking, and obesity have been strongly correlated with elevated SDF. The mechanisms of how these occur can be a combination of increased ROS and apoptosis to heat stress and other mechanisms that are still unknown [26]. Alcohol consumption also has been shown to have deleterious effects on sperm parameters though much is still unknown about SDF [32-34] In a meta-analysis was concluded that alcohol’s effects on sperm parameters were dependent on the frequency of consumption, and there were significant correlations with daily alcohol consumption and SDF, whereas occasional intake had no correlation with SDF [32]. SDF levels can change rapidly based on the health and lifestyle factors even with the length of time between ejaculations. The abstinence period, longer than the recommended 2-5 days, will increase SDF as sperm in the epididymis are exposed to ROS [1-3].
In addition to these lifestyle factors and exposures, the way the sperm is handled in the IVF laboratory can also greatly increase SDF. Determined that SDF levels significantly decreased after processing over gradient, but the SDF index would increase to abnormal levels after two hours of incubation or 1.5 hours of exposure to Poly Vinyl Pyrrolidone (PVP) [35]. In timing and temperature, though not cryopreservation methods, in the laboratory had great effects on inducing further SDF [36]. These different sources and causes of oxidative stress make it clear why SDF is so prevalent and an area that merits more investigation. Microfluidic technology uses the sperm’s motility to propel it through a series of micro channels to ideally select out the best of the cohort based on its own moment and morphology simulating the female reproductive tract’s natural ability to sort sperm [37]. Motility and morphology near 100% following the use of microfluidic technology. However, this technology has not been widely adopted for clinical use. More recent development of a macro-microfluidic chamber that contains a polycarbonate filter of different pore diameters act as barriers which only allow sperm with adequate motility and morphology to pass. Recent publications have shown that the sperm sorted through this device have nearly undetectable DNA fragmentation with significant improvements over the neat semen sample as well as gradient processed [38,39]. The commercially available device used in these studies has the potential to change the methods of sperm processing by making a device accessible, affordable and easy to use in the laboratory. The device also eliminates the use of centrifugation, which is beneficial to eliminating a step known to introduce reactive oxygen species, but could also eliminate the need for the expensive equipment. More clinical studies that show the effects on improving IVF outcomes, particularly in men with high SDF, are needed.
Institutional Review Board approval was received (IRB# 17-11-EX-0222) before the beginning of the study. These data were collected as part of a prospective double-blinded study evaluating couples that were undergoing IVF and planned to utilize PGT-A to evaluate the effects of sperm DNA fragmentation on outcomes at Midwest Fertility Specialists. Patients consented for the study were using fresh ejaculated sperm and were asked to answer a lifestyle questionnaire regarding alcohol consumption, smoking status, heat and living exposures, body mass index, age, and whether they consumed any daily vitamins or supplements. Sperm was collected by masturbation in a sterile collection cup. The semen sample was delivered to the IVF laboratory and placed on a warmer at 37°C for liquefaction for 30-60 minutes. A basic semen analysis was performed measuring the sperm count (millions/mL), percent motile, volume, viscosity, and the presence of either white blood cells or sperm agglutination. A visual assessment of the morphology was documented as normal or abnormal by the same embryologist, the principal investigator, for all samples. Three vials with random numbers were selected and documented for each semen sample. An aliquot of the raw semen, approximately 0.5 mL, was placed in the first vial and flash frozen in liquid nitrogen. The remaining semen was layered over a two-layer gradient (Isolate, Irvine Scientific) for centrifugation at 3000 g for 15 minutes. The supernatants were removed and the pellet washed for an additional 5 minutes. The supernatant was removed. The pellet was resuspended with givf plus to achieve a final concentration of approximately 2 million motile sperm per ml. An aliquot of this sample was placed in the second vial labeled with a random number and flash frozen in liquid nitrogen. The processed sperm sample was then placed in the incubator set at 7.3% carbon dioxide and 5% oxygen until the time of ICSI or insemination. The randomly labeled vials were sent in batches on dry ice for sperm DNA fragmentation assay (SDFA, as in SCSA®, ReproSource, Woburn MA). For each sample, the abstinence period and hours of incubation post processing were recorded. All data from the study was double blinded. The unblinding occurred after the study was closed to ensure no bias on any type of the data collected. The samples from the main study were only analyzed if adequate sperm counts were present and all three time points could be frozen for analysis. Very low sperm counts, previously frozen sperm samples, and surgically derived sperm were excluded from this study.
Institutional Review Board approval was received (IRB# 18-10-NH-0229) for a follow up study comparing different preparation methods on the same ejaculate from 30 different semen analysis patients to determine which method best improved sperm quality. All samples in this portion of the study were labeled with a random number for blinding and sent in batches on dry ice for sperm DNA fragmentation assay (SDFA, as in SCSA®, ReproSource, Woburn, MA). An aliquot of 0.3 mL of neat semen was frozen and the remainder of the semen allocated between 5 preparation methods [1-5]. A sterile syringe was used to load 0.8 mL of raw semen in a ZyMōt Sperm Separation Device (1,DxNow), then layered with GIVF+ and incubated for 30 minutes. At the end of incubation, 0.3 mL of sperm was removed from the out port and frozen. Approximately 0.8 mL of semen was layered over the Isolate gradient (2,Irvine) and SpermGrad gradient (3,Vitrolife) per package insert and centrifuged for 15 minutes. The samples were reconstituted with GIVF+ and an aliquot of each was frozen. The pellet was re-formed and the tube incubated at an angle to obtain the Swim-Up (SU) Isolate+SU (4) and Spermgrad+SU (5), for each gradient. After one hour, an aliquot from the top of the tube was frozen. Each blinded sample, 6 per semen sample, was analyzed using the Sperm DNA Fragmentation Assay (acridine orange/flow cytometry SDFA™) and the OSA™ test which directly measures sperm damage from oxidative stress by quantifying the presence of “adducts,” molecules in semen covalently modified by free radicals/reactive oxygen species. The patient’s age, count, motility, days of abstinence, and volume were recorded. Statistical Analysis System (SAS) was performed using John's Macintosh Project (JMP) (SAS 2018) and statistical tests were considered significant at p=0.05.
Statistical analysis
Analyses were performed using JMP (SAS 2018) or Excel (Microsoft 2018). Box and whisker plots were created to compare DFI, OSA, and HDS for different time points of the sperm samples in the first portion of the study and to compare different preparation methods in the second part of the study. Wilcoxon rank sums tests for nonparametric data was used to analyze the DFI, OSA, and HDS by lifestyle factor reported in the study. Bivariate analysis was used to analyze continuous data such as age and Body Mass Index (BMI).
The data from 133 fresh ejaculations, collected for Invitro Fertilization (IVF) on the day of the oocyte retrieval, were used in the first part of the study, and 30 total fresh ejaculations, all different patients collected at the time of routine semen analysis, were included in the second part of the study. Statistics were analyzed based on the neat semen as well as the processed sample used for IVF Insemination (INSEM). The neat DFI was not correlated with age, morphology, or oligospermia status (<20 million/mL). The motility of the neat sample was negatively correlated with DFI (p<0.0001). Neat sperm count was neither correlated with neat DFI nor INSEM DFI (p=0.4318 and p=0.1302, respectively). Men that consumed alcohol daily trended towards a higher DFI than those that drank multiple times per week and significantly higher than those who never drink (p=0.0608 and p=0.0290, respectively), but interestingly not those who drank rarely. DFI was also positively correlated with OSA and HDS in the neat and processed sample (INSEM). The DFI of the INSEM sperm sample was positively correlated with age, poor morphology, and oligospermia (p=0.0208, p<0.0001, p=0.0006, respectively. There was no correlation with BMI or smoking status in either group. The processing method of gradient and wash was effective for most patients with an overall 40.2% decrease in DFI, 27.3% improvement in OSA, and a 38.6% decrease in HDS. However, 15.8% men had an increase in DFI and 20.3% had an increase in OSA from the neat semen sample to the sample used for IVF (Tables 1-4).
| Alcohol consumption | Number | Neat DFI | Insemination/post processing DFI |
|---|---|---|---|
| Daily | 24 | 24.99 ± 12.1 | 17.16 ± 16.3 |
| Multiple Times/Week | 53 | 19.48 ± 10.3 | 8.8 ± 11.7 |
| Rarely | 17 | 17.22 ± 9.5 | 7.69 ± 9.3 |
| Never | 34 | 20.11 ± 12.9 | 22.16 ± 12.0 |
Note: DFI=DNA Fragmentation Index.
Table 1: Patient alcohol consumption reported by patient survey and DNA Fragmentation Index (DFI).
| Group 1 | Group 2 | DNA Fragmentation Index (DFI)(p-value) | Oxidative Stress Adducts (OSA)(p-value) | High DNA Stainability (HDS)(p-value) |
|---|---|---|---|---|
| Daily | Multiple/Week | 0.0608 | 0.5975 | 0.0549 |
| Multiple/Week | Rarely | 0.9826 | 0.6385 | 0.941 |
| Rarely | Daily | 0.1108 | 0.439 | 0.1485 |
| Never | Daily | 0.0290 | 0.7709 | 0.0567 |
| Never | Rarely | 0.5161 | 0.3476 | 0.8572 |
| Never | Multiple/Week | 0.4679 | 0.5065 | 0.9182 |
Table 2: Comparisons between groups of alcohol consumption on the neat semen sample.
Increasing numbers of studies are showing the important impact that sperm DNA fragmentation has on infertility amongst couples, as well as their impact on IVF outcomes. However, many studies have failed to look at outcomes based on the DFI of the sample used for treatment, instead evaluating outcomes based on a previously analyzed ejaculate from another period of time. The quality of sperm may vary ejaculation to ejaculation in a very short period of time deeming this data inaccurate. In the first portion of the study, we analyzed the differences in DFI, OSA and HDS after processing and at the time of insemination. The gradient and wash did improve most sperm samples but there were 15% of samples in which the DFI was higher after processing with a similar trend for OSA. The DFI of the neat ejaculate was not correlated with age, morphology, oligospermia diagnosis, or smoking status. The motility was negatively correlated with neat DFI, and DFI was positively correlated with OSA and HDS. The sperm count of the neat sample was not predictive of the neat or INSEM DFI.
This information is important for the argument of performing routine SDF testing on all patients prior to treatment because very little was able to predict the health of the sperm based on DFI, HDS, and OSA levels (Figures 1-4).
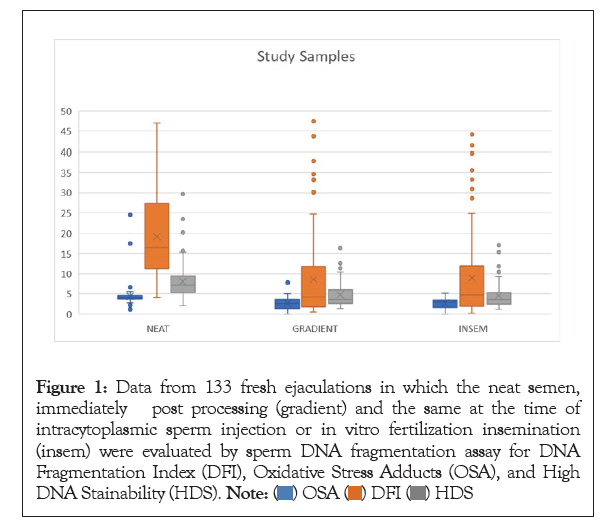
Figure 1: Data from 133 fresh ejaculations in which the neat semen, immediately post processing (gradient) and the same at the time of intracytoplasmic sperm injection or in vitro fertilization insemination (insem) were evaluated by sperm DNA fragmentation assay for DNA Fragmentation Index (DFI), Oxidative Stress Adducts (OSA), and High DNA Stainability (HDS). 
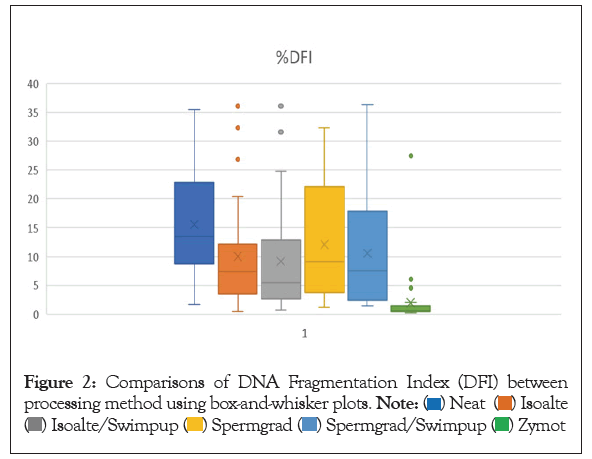
Figure 2: Comparisons of DNA Fragmentation Index (DFI) between processing method using box-and-whisker plots. 

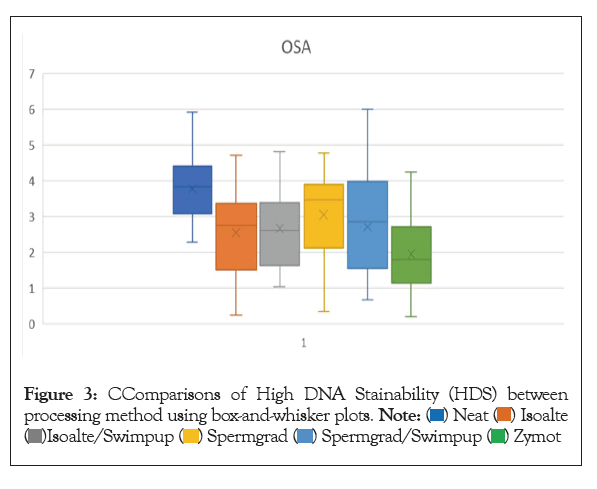
Figure 3: CComparisons of High DNA Stainability (HDS) between processing method using box-and-whisker plots.

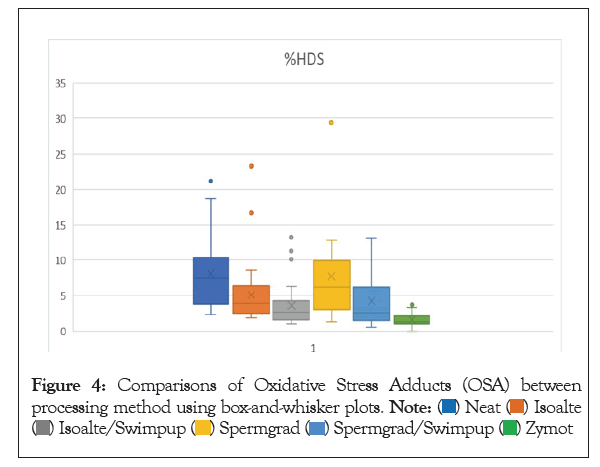
Figure 4: Comparisons of Oxidative Stress Adducts (OSA) between processing method using box-and-whisker plots. 

| Group 1 | Group 2 | DFI (p-value) | OSA(p-value) | HDS(p-value) |
|---|---|---|---|---|
| Daily | Multiple/Week | 0.0034 | 0.0290 | 0.0729 |
| Multiple/Week | Rarely | 0.0562 | 0.2508 | 0.3341 |
| Rarely | Daily | 0.2028 | 0.5123 | 0.3639 |
| Never | Daily | 0.0192 | 0.5875 | 0.1491 |
| Never | Rarely | 0.1619 | 0.5895 | 0.5421 |
| Never | Multiple/Week | 0.8479 | 0.3043 | 0.9127 |
Table 3: Comparisons between groups of alcohol consumption on the processed sperm sample at the time of intracytoplasmic sperm injection or in vitro fertilization insemination.
| Preparation method 1 | Preparation method 2 | DFI (p-value) | OSA (p-value) | HDS (p-value) |
|---|---|---|---|---|
| Neat | Isolate | 0.0052 | 0.0002 | 0.0011 |
| Neat | Isolate+SU | 0.0023 | 0.0002 | <0.0001 |
| Neat | SpermGrad | 0.074 | 0.0574 | 0.2837 |
| Neat | SpermGrad+SU | 0.0184 | 0.0024 | 0.0002 |
| Neat | DxNow | <0.0001 | <0.0001 | <0.0001 |
| Isolate | Isolate+SU | 0.6789 | 0.836 | 0.0389 |
| Isolate | SpermGrad | 0.375 | 0.0656 | 0.0326 |
| Isolate | SpermGrad+SU | 0.652 | 0.7394 | 0.1808 |
| Isolate | DxNow | <0.0001 | 0.0657 | <0.0001 |
| SpermGrad | SpermGrad+SU | 0.4464 | 0.3183 | 0.0044 |
| SpermGrad | Isolate+SU | 0.1761 | 0.0656 | 0.0005 |
| SpermGrad | DxNow | <0.0001 | 0.0004 | <0.0001 |
| DxNow | Isolate+SU | <0.0001 | 0.0224 | <0.0001 |
| DxNow | SpermGrad+SU | <0.0001 | 0.0428 | <0.0001 |
| SpermGrad+SU | Isolate+SU | 0.4779 | 0.9764 | 0.0044 |
Note: Wilcoxon Rank Sums Test used to analyse the means between each preparation method.
Table 4: DNA Fragmentation Index (DFI), Oxidative Stress Adducts (OSA), and High DNA Stainability (HDS) compared between preparation methods on the same ejaculate.
Interestingly, the INSEM DFI was positively correlated with age, poor morphology, and oligospermia. However, it was not predicted by the original sperm count, BMI, or smoking status. BMI and smoking have been shown to be strong predictors by other studies; however, only 6 patients reported being smokers in our study, and BMI was not correlated either by individual or when grouped by percentage ranges. The correlation with daily alcohol consumption and DFI with both the neat and INSEM sperm sample was interesting and in agreement with previous reports [32]. Alcohol intake is rarely discussed with the male partner during the fertility process, and from our data, consumption should be reduced during fertility treatments. Further studies should be performed with more controlled conditions to understand the effects of health, lifestyle, environment, and age on overall sperm health and if modifications to these could alter the fertility status of both the male and the couple.
DFI, OSA, and HDS were all positively correlated even after the processing. It is of notable importance because the traditional gradient method did not always effectively reduce the sperm damage from oxidative stress as measured by OSA. Furthermore, HDS is a measurement of sperm that are likely either immature or have a high histone retention and this level was also not effectively reduced by the gradient method. This study demonstrated that some semen samples can be effectively processed in the IVF laboratory to reduce DFI, but others may need more aggressive options. More studies should be done to determine if reducing the DFI at the time of insemination could reduce the known impact of SDF on fertility outcomes. During fertilization, changes in the sperm, such as oxidative stress disrupting lipid membrane and acrosome function, can greatly disrupt the sperm’s ability to fertilize the oocyte. In sperm with high histone retention, which can be caused by oxidative stress, the sperm proteome can be greatly altered. This can have severe implications on the subsequent embryo and offspring [40-43]. Studies are showing alterations in sperm gene expression are correlated with the similar correlations as SDF with fertilization, embryo development, implantation, and live birth rates [41-50]. The mechanism of SDF may be alterations of gene expression by fragments across key genes or the maintenance of histones across key developmental regions that the oocytes are unable to reconstruct at fertilization. Furthermore, breaks in the DNA strands of the sperm around the centrosome, the organizing center of the spindle, may affect fertilization. The centrosome is inherited from the sperm and is key in pronuclear formation following fertilization [51]. DNA breaks may interfere with this key step causing failed fertilization and subsequent development.
The second part of the study demonstrated that a sperm separation device could improve DFI, HDS, and OSA over other gradient and gradient followed by a swim up. Interestingly, the swim up did not improve these parameters over a gradient as previously reported [35,52]. There have been some attempts at methods to select the best quality sperm with good DNA integrity, but these have not become popularly used for reasons such as complexity of equipment, cost, inconvenience, or the lack of consistent results. The use of Hyaluronan Acid (HA) binding technology, marketed as PICSI dishes, uses hyaluronan strips that mature sperm are most likely to bind to due to alterations in the plasma membrane. Studies have shown that sperm that bind to these strips of HA have decreased aneuploidy, better DNA integrity, and fewer apoptotic markers. The use of this technology has improved IVF outcomes and implantation rates [53-57]. Despite these results, the PICSI dishes are not widely used. Hypo-osmotic swelling is an older, simpler method that has been reported to select sperm with minimal DNA fragmentation and could be a viable option for ICSI selection [58]. However, this method has not gained popularity. Other more advanced techniques that have shown promise but are still not widely used are Magnetic-Activated Cell Sorting (MACS), zeta potential technique, and electrophoresis cell sorting [59]. MACS is a method that selects sperm based on the early signs of apoptosis by the presence of phosphatidylserine in the plasma membrane. Paramagnetic microbeads bind to the sperm with phosphatidylserine present and then are exposed to a magnetic field allowing all the unbound sperm to pass. The method, particularly in combination with traditional density gradient separation, is effective at reducing SDF, improving morphology, and decreasing apoptotic markers [59-61]. Sperm separated by MACS have also been shown to have more optimal protamine and acrosome content showing promise at utilizing MACS separated sperm for intrauterine insemination or traditional IVF [62]. However, very little data exists on the clinical utility of this method. The Zeta potential technique works on the assumption that a mature sperm has a negatively charged plasma membrane. The sperm are exposed to a positively charged centrifuge tube and any sperm or cell that does not bind to the charged tube is eliminated. The process has been shown to elucidate sperm with better DNA integrity, morphology, and motility compared to traditional methods [63]. The major drawback to this method is that it must be done relatively quickly after ejaculation because this charge is lost as the sperm undergoes capacitation and is ineffective on previously frozen sperm due to a decrease in the negative charge during cryopreservation [63,64]. Electrophoresis as a method of sperm sorting has shown to improve sperm quality compared to the neat semen sample; however, it does not show any improvement over traditional gradient separation and involves complex technique and equipment for no real added benefit [65] Recently, the use of the sperm separation device used in this study has been reported to be effective at improving sperm quality and outcomes [66-72]. The advantages of using this device are that it is simple to use, cost effective, disposable, and eliminates the centrifugation step. In findings not yet published in our laboratory, we found that the DFI, OSA, and HDS at the time of ICSI or IVF insemination was significantly correlated with fertilization rates. The routine use of this novel separation device could improve fertilization and possibly other subsequent outcomes. In recent years, patients with high SDF and typically failed previous cycles have been undergoing surgery to obtain testicular sperm with better DNA integrity to improve IVF outcomes. The use of this device could be an alternative and safer, less expensive method for some patients and could arguably improve outcomes for all patients by improving sperm quality.
Previous studies have shown correlations between DFI and semen parameters as well as patient age and lifestyle factors. This study took it a step further to investigate correlations that might exist between these factors and the processed sperm used for IVF. The DFI of the INSEM sperm sample, processed using gradient and wash in preparation for standard IVF insemination and ICSI, was positively correlated with age, poor morphology, and oligospermia. There was no correlation with BMI or smoking status in the neat or processed sperm samples. The processing method of gradient and wash was effective for most patients with an overall 40.2% decrease in DFI, 27.3% improvement in OSA, and a 38.6% decrease in HDS. However, 15.8% men had an increase in DFI and 20.3% had an increase in OSA from the neat semen sample to the sample used for IVF. This study also compared the SDFA results for several different methods available for sperm processing: gradient, swim up, gradient followed by swim up, and Zymot. Based on these findings, Zymot shows promising results to improve the quality of sperm post processing.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref ] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Broussard AL, Leader B, Tirado E, Russell H, Beydoun H, Colver R, et al. (2023) Lifestyle Factors and Laboratory Sperm Processing Techniques Correlation with Sperm DNA Fragmentation Index, Oxidative Stress Adducts and High DNA Stainability. J Tourism Hospit.12:292.
Received: 15-Jun-2023, Manuscript No. ANO-23-25098; Editor assigned: 19-Jun-2023, Pre QC No. ANO-23-25098 (PQ); Reviewed: 03-Jul-2023, QC No. ANO-23-25098; Revised: 10-Jul-2023, Manuscript No. ANO-23-25098 (R); Published: 17-Jul-2023 , DOI: 10.35248/2167-0250.23.12.292
Copyright: © 2023 Broussard AL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited