Drug Designing: Open Access
Open Access
ISSN: 2169-0138
ISSN: 2169-0138
Research Article - (2021)Volume 10, Issue 5
ZingiVir-H, a herbo-mineral Ayurvedic medicine targets for managing Viral fevers, Bronchitis and Acute Respiratory tract infections. It is formulated using five potent herbs and two purified heavy metals (Mercuric sulphide-HgS and Arsenic trisulphide-As2S3). The toxicity associated with metallic constituents was removed by subjecting to various detoxifying processes as described in the century-old classical literatures of Ayurveda. However, the objection raised against metal-derived drugs of Ayurveda is the lack of empirical evidence for its claimed non-toxicity after the detoxification process. Considering the widespread misconception, we have projected this study to assess the cellular toxicity of ZingiVir-H, and its two-heavy metal ingredients (raw and at various stages of detoxification steps) in the L6 rat myoblast cell line based on MTT assay and ROS production. In MTT assay a substantial reduction was reflected in the cellular toxicity for purified HgS and As2S3 when compared to their raw forms. Moreover, ZingiVir-H has also recorded non-toxicity especially in low concentrations. Alternatively, purified HgS and As2S3 at 20 μg/mL also recorded a significant reduction in ROS production when compared to that of raw HgS and As2S3. Finally, ZingiVir-H and the drug without its metal constituents recorded only 4.5 ± 0.33% and 1.1 ± 0.23% of ROS. These results established that the detoxification of HgS and As2S3 leads to a significant reduction of toxicity as well as the ROS production. Further, the quantitative analysis by X-ray photoelectron spectroscopy (XPS) evidently revealed that the percentage of arsenic, mercury and sulphur was significantly reduced after the detoxification/purification of HgS and As2S3. In light of our evaluation, we hope this research paper will attract a wide attention to confirm the toxicity threshold of herbomineral drugs and purified heavy metals as per Ayurvedic principles through cytotoxicity assessment.
Cytotoxicity; ZingiVir-H; Heavy metals; Ayurvedic detoxification; Reduced toxicity; Ayurveda drug
The use of heavy metals in traditional systems like Ayurveda and Siddha sparks a widespread concern about their toxicities and adverse health impacts. Unfortunately, the most lamentable candidate is Bhasma, where its preparation methods have been criticized and disparaged by the scientific world for its toxic heavy metal ingredients like lead, mercury, arsenic etc [1,2]. In this current era of scientific validation and various good manufacturing practices, the minerals and metals that are transformed into drugs should be non-toxic and maintain excellent quality, safety and therapeutic efficacy. The metallic and mineral preparations used in the Ayurvedic drugs have a unique and indigenous preparation procedure which involves Shodhana (detoxification and purification) and Marana (incineration and calcination) and this has been used for several millennia in clinical practices in India. The practitioners of this science have developed these detoxification methods to detoxify the raw metallic/minerals through chemical transformations and modify their therapeutic attributes to enhance potency [3,4]. Extensive use and applications of these purified metallic and mineral formulations over decades without any menacing effects are the best proof of their therapeutic efficacy and safety, as evidenced by several years of experiences [5]. Inadequate preclinical and clinical data to support these claims impede the public acceptance of the Ayurvedic formulations. Hence, it is a growing demand of the time for empirical preclinical and clinical evidences of all the metallic/mineral Ayurvedic formulations to prove that they are safe and effective in disease management [5,6].
Pankajakasthuri Herbals India Pvt. Ltd., Poovachal, Thiruvananthapuram, Kerala has formulated the ZingiVir-H tablets (Drug License Number: DL. NO: 50/25D/96) recently based on a proven traditional herbo-mineral Ayurvedic formulation that has significant antiviral and antipyretic properties [7]. In Ayurveda, preparations involving heavy metals are called ‘Bhasmas’ (ash), and which have been in use since ancient times [8]. These heavy metals in combination with specific herbal constituents form an effective medicine in disease management. The ZingiVir-H tablets were originally developed to combat fever, various viral infections, bronchitis and Acute Respiratory Tract Infection (ARTI). This herbo-mineral formulation contains Eugenia caryophyllus, Zingiber officinale, Cyperus rotundus, Hedyotis corymbosa, Apium graveolens along with processed and purified Chayilyam (HgS) and Thalakam (As2S3) as per the protocols prescribed in the classics of Ayurveda (Table 1). The HgS and As2S3 in their virgin forms obtained from ores are impure and toxic. These impurities are removed by a stringent process called shodhana (detoxification), where the heavy metals were exposed to different detoxification steps like purification, trituration and heating. The study drug ZingiVir-H has also underwent through the same Shodhana process to negate its evident toxicities, thus making the drug clean and safe in applications.
| SL. No. | Ingredients | Form used | Quantity/500 mg |
|---|---|---|---|
| 1 | Eugenia caryophyllus | Dried clove bud powder | 55 mg |
| 2 | Zingiber officinale | Fresh ginger juice or aqueous extract | 200 mg |
| 3 | Cyperus rotundus | Dried roots and rhizomes powder | 35 mg |
| 4 | Hedyotis corymbosa | Dried whole plant powder | 30 mg |
| 5 | Trachyspermum ammi | Dried fruit powder | 60 mg |
| 6 | HgS (Mercuric sulphide) | Purified and processed as per texts | 20 mg |
| 7 | As2S3 (Arsenic trisulphide) | Purified and processed as per texts | 10 mg |
| 8 | Starch | - | 90 mg |
Table 1: Ingredients used in the preparation of ZingiVir-H tablets.
Since it is well reported that the heavy metals induce cytotoxicity in cell lines, the present study aimed to assess the cellular toxicity of ZingiVir-H tablets as well as its heavy metal ingredients HgS and As2S3 (raw and at various stages of detoxification steps) in the L6 rat myoblast cell line based on MTT assay and ROS production to confirm that toxicity has been reduced during the detoxification processes.
Chemicals and reagents
Dulbecco’s Modified Eagle’s Medium (DMEM), Dimethyl s u l foxide,3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Fetal Bovine Serum (FBS) and 2,7-Dichlorodihydrofluorescin Di-Acetate (DCFH-DA) were purchased from Sigma-Aldrich Chemicals (St Louis, MO, USA). Trypsin-EDTA and Antibiotic-antimycotic were purchased from Gibco Invitrogen (Carlsbad, CA, USA). Hydrogen peroxide (H2O2) was purchased from Merck. All the chemicals and reagents used in this study were analytical grade and maintained high quality.
Detoxification (Shodhana) of mercuric sulphide
The mercuric sulphide was grounded with an equal quantity of lime powder in a khalwa yantra (Mortar and Pestle) for 3 days. Then the resultant mixture was filtered through double layered cheesecloth. The filtered mercuric sulphide was subsequently triturated along with an equal quantity of Allium sativum and half the amount of rock salt. The grinding was carried out until the mixture in the khalwa yantra turned into a black colored paste. The mixture was then washed with hot water or sour fermented liquid to separate elemental and inorganic mercury. The black paste was then washed off and mercury was eliminated. The process of washing and decanting/separation was performed several times until the entire mercury is separated. Eventually, non-toxic mercuric sulfide was earned, and which was then procured for the final formulation of ZingiVir-H [9,10].
Detoxification (Shodhana) of arsenic trisulphide
Arsenic was grinded into small pieces and bundled in a kora cloth to prepare a cloth bundle. The cloth bundle was suspended in an earthen pot to make a dolayantra (apparatus used for steam boiling). The steam boiling was performed in any of the following liquid media like aqueous extract of Deninca sahispida, water extract using ash of Seasamum indica or lime water over mild fire preferably for the duration of 3 hours. After that, the cloth bundle was taken out and pieces of arsenic were collected. Subsequently, the gathered arsenic was washed with lukewarm water and dried. This process eventually removed the toxicity of arsenic, and the pure arsenic granules were then taken for medicinal preparation [11,12].
Quantitative analysis of test samples for the presence of heavy metals by XPS
The quantitative analysis of arsenic, mercury and sulphur content present in raw HgS and As2S3, after its purification/ detoxification process and final product, i.e. ZingiVir-H tablets, was determined using X-ray photoelectron spectroscopy (XPS) (PHI 5000 Versaprobe, USA). A focused monochromatized Al Kα X-ray source (hν=1486.6 eV) was used at 15 kV. The analyzed area of the sample was 200 μm in diameter. For charge correction, binding energies were calibrated using the C 1s peak from adventitious carbon with a fixed value at 284.8 eV. The PHI Multipak software processed spectroscopic data.
Samples tested
Sample 1: Raw mercuric sulphide-HgS (Chayilyam)
Sample 2: Raw arsenic trisulphide-As2S3 (Thalakam)
Sample 3: HgS after 1st stage detoxification
Sample 4: As2S3 after 1st stage detoxification
Sample 5: HgS and As2S3 in 2:1 ratio (i.e. 20 mg HgS and 10 mg As2S3) after final detoxification
Sample 6: ZingiVir-H tablets containing 2:1 ratio of HgS and As2S3
Sample 7: ZingiVir-H tablets without HgS and As2S3
Sample 8: Hydrogen peroxide-H2O2 (Positive control)
Sample 9: Growth medium alone (Negative control)
Cell culture conditions: L6 rat myoblast cell line was obtained from NCCS, Pune, India. The cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% Fetal Bovine Serum (FBS) and 1% antibiotic-antimycotic solution (Streptomycin). Cells were nurtured in humidified conditions at 37°C, 5% CO2 [13].
Cytotoxicity testing of test samples by MTT ASSAY in L6 cells: The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay is a colorimetric assay based on the conversion of MTT into formazan crystals by living cells, which determine the mitochondrial activity [14]. Since, for most cell populations, the total mitochondrial activity is related to the number of viable cells, this assay is broadly used to measure the in-vitro cytotoxic effects of chemicals on primary cells. This assay measures the reduction of yellow MTT by mitochondrial succinate dehydrogenase. The MTT enters the cells and passes into mitochondria where it will get reduced to an insoluble, colored (dark purple) formazan product. The cells are then solubilized with an organic solvent and the released, solubilized formazan reagent is measured spectrophotometrically. Since, the reduction of MTT can only occur in metabolically active cells, the level of activity is a measure of the viability of the cells.
L6 cells were trypsinized and seeded in 96 well-plates. After the attachment of cells, different concentrations of test samples (2.5 μg/mL, 5 μg/mL, 10 μg/mL, 20 μg/mL and 50 μg/mL) were treated and incubated at 37°C for 24 h. Sample-free growth media was used as the negative control. The treated media was removed after incubating for 24 h. Thereafter, 100 μL MTT reagent (50 μg/well) was added and then incubated for another 4 h in the dark. Subsequently, after the incubation process, the reagent was removed and 200 μL DMSO was added to all wells. The plates were then wrapped with aluminum foil and agitated in a shaker for 45 min. Following this, absorbance was read at 570 nm using a multimode reader. The assay was carried out in triplicates and the percentage of cell viability was calculated using the following formulae.
Percentage viability=(OD of test/OD of control) × 100
Detection of intracellular Reactive Oxygen Species (ROS) after the treatment with test samples by H2DCF-DA staining: Intracellular ROS levels were estimated using DCFH-DA staining [15]. The cells were seeded at a density of 1 × 103 cells/well in 6 well-plates for ROS assay. The cells were then allowed to differentiate into myotubes, and the resultant cells were further exposed to various sub-toxic concentrations (20 μg/mL) of test samples for 24 h. After this treatment, cells were incubated with DCFH-DA for 20 minutes and then underwent PBS washing. Quantification of ROS production by cells was determined by measuring the intracellular DCF fluorescent intensity by flow cytometer (BD FACS Aria II, BD Bioscience, and USA).
Statistical analysis: For cytotoxicity analysis, the data from 4 to 5 separate cultures were averaged to calculate means and standard deviation. The ROS analysis value was obtained by calculating the average of two separate cultures. SPSS (version 16) statistical software was used for the data analyses and p<0.05 was taken as significance level.
Quantitative analysis of test samples
The quantitative analysis evidently revealed that the percentage of arsenic, mercury and sulphur significantly reduced after the detoxification/purification of HgS and As2S3. Finally, a very low percentage of arsenic, mercury and sulphur was recorded in the final product i.e., ZingiVir-H tablets when compared to that of raw As2S3 and HgS (Table 2).
| Parameters | Heavy metal content (%) | |||
|---|---|---|---|---|
| Raw As2S3 | Raw HgS | Intermediate step during the detoxification of As2S3 and HgS | ZingiVir-H tablets | |
| Arsenic | 40.75 ± 1.02 | 2.50 ± 0.4 | 12.19 ± 1.09 | 1.53 ± 0.40 |
| Mercury | 0.41 ± 0.05 | 73.26 ± 2.14 | 19.99 ± 2.2 | 5.44 ± 0.844 |
| Sulphur | 32.8 ± 1.477 | 13.49 ± 1.18 | 12.50 ± 1.54 | 2.69 ± 0.30 |
Table 2: Quantitative analysis of test samples for the presence of heavy metals by XPS.
MTT assay: The cellular viability of test samples was analyzed by MTT assay on L6 cell lines. The OD value of control cells (test sample-free) was taken as 100% and then calculated the percentage of reduction of OD in samples exposed cell lines. The samples 1 and 2 have recorded significant cytotoxicity when compared to the other four samples tested (samples 3, 4, 5 and 6) (Figure 1). From the MTT results, it was clear that the purified HgS and As2S3 didn’t record any toxicity at low concentrations when compared to that of raw HgS and As2S3. However, minimal toxicity indications were observed for the purified HgS and As2S3 at higher concentrations. Finally, ZingiVir-H has recorded non-toxicity to the tested cell line at low concentrations (Figure 2). The cytotoxicity of ZingiVir-H in higher concentrations was significantly less than the raw HgS and As2S3. In accord to this, ZingiVir-H without its ingredients HgS and As2S3 has also recorded non-toxicity in the test cell line. This eventually proved that the herbal parts used for the formulation of ZingiVir-H tablets didn’t have toxicity. These results proved that purified HgS and As2S3 used for formulating ZingiVir-H tablets were responsible for reducing cellular toxicity.
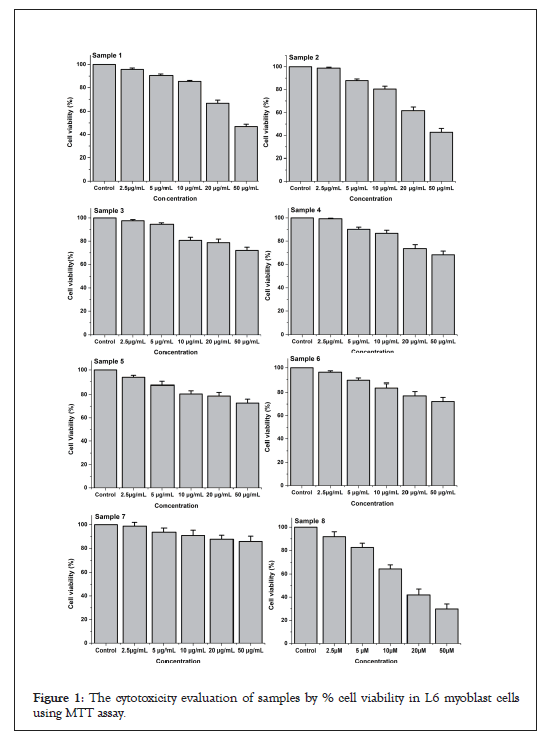
Figure 1: The cytotoxicity evaluation of samples by % cell viability in L6 myoblast cells using MTT assay.
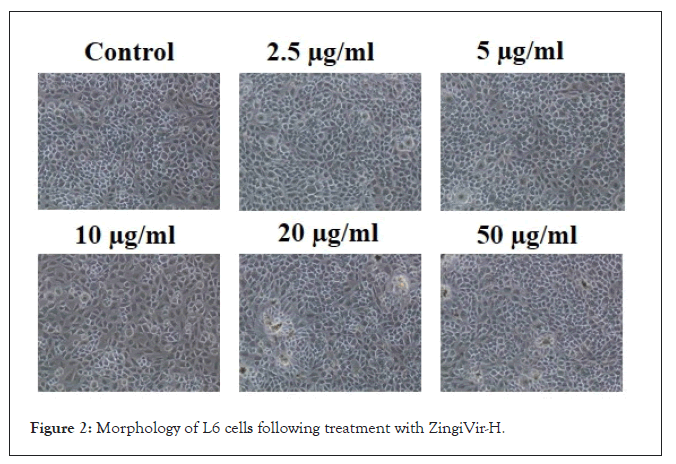
Figure 2: Morphology of L6 cells following treatment with ZingiVir-H.
ROS: The intracellular ROS production was quantified by measuring the fluorescence of DCF by flow cytometer (BD FACS Aria II, BD Bioscience USA). Samples 1 and 2 have recorded an increase in ROS production of 35.2 ± 5.65% and 24.4 ± 0.77% at 20 μg/mL. The samples 3 to 5 at 20 μg/mL recorded significantly reduced ROS production when compared to that of samples 1 and 2. Finally, sample 6 i.e., ZingiVir-H has recorded only 4.5 ± 0.33% of ROS, which was very less when compared to that of other tested samples except sample 7 i.e. ZingiVir-H without HgS and As2S3. The ZingiVir-H without HgS and As2S3 recorded only 1.1 ± 0.23% ROS production. This result indicated that ZingiVir-H effectively inhibited the ROS production in L6 cells when compared to the raw HgS and As2S3. The significant reduction in the ROS in ZingiVir-H treated cells may be due to the presence of various antioxidant phytochemical ingredients (Figure 3).
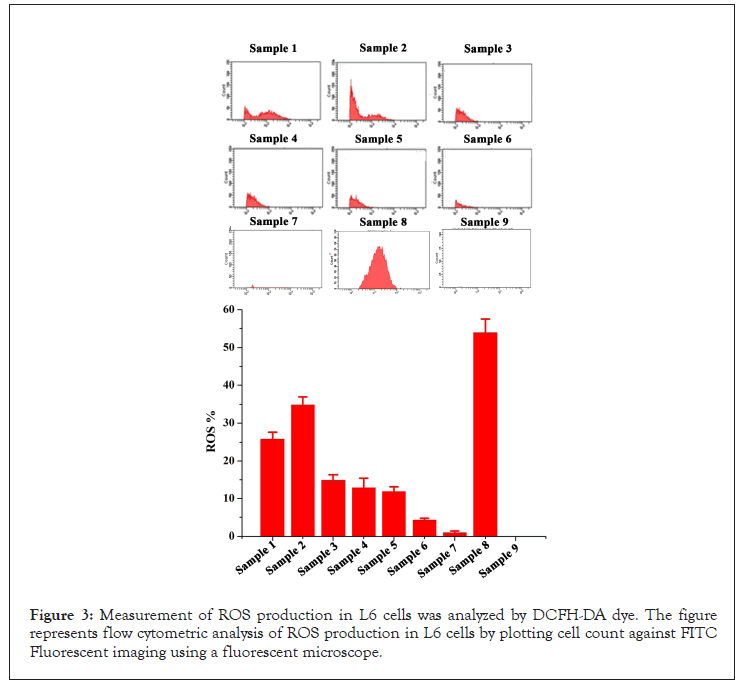
Figure 3: Measurement of ROS production in L6 cells was analyzed by DCFH-DA dye. The figure represents flow cytometric analysis of ROS production in L6 cells by plotting cell count against FITC Fluorescent imaging using a fluorescent microscope.
According to a World Health Organization (WHO) report, herbal and herbomineral medicines are often used by several individuals in developing nations to treat a variety of ailments [16]. Unlike western chemotherapeutic agents, most individuals believe that herbal medications are safe and non-toxic [17]. Therefore, common people usually use herbal medicine for protracted periods to achieve a desirable effect. Conversely, it has been reported that several herbal medicines used in the Indian subcontinent and China contain a higher concentration of heavy metals than in other geographical regions [18,19]. A study showed that, one of five Ayurvedic herbal medical products produced in South Asia contains high levels of lead, mercury, arsenic etc. [20]. These heavy metals are integral to some formulations and are used in medications for several centuries [21]. Ayurvedic formulations containing heavy metals are produced from raw materials strictly after different processes like detoxification, purification (shodhana) trituration and heating. The classical Ayurvedic textbooks cited special notes on the toxicity of heavy metals and recommend the special pharmacological process to detoxify them before using them as medicine. The metals in their virgin form obtained from ores may contain several impurities. These impurities are removed by the special Shodhana process which removes the unwanted part from the raw material and separates impurities. Consequently, components found in the final products do not produce any hazardous effects. According to several scientific reports, the toxicity of heavy metals decreases significantly after detoxification. The reduction in the toxicity was mainly confirmed through the animal experiments where no significant change in the histopathology of the vital organs was recorded after the treatment with metallic/mineral herbal formulations prepared from purified metals [17,22-26].
Ayurvedic textbooks highlight the role of heavy metals in the proper function of the human body, especially maintaining the metabolic equilibrium. These metals include mercury, gold, silver, copper, iron, tin, lead, zinc etc. This equilibrium state provides the basis for strong immunity to the body [27]. Therefore, any imbalance in the composition of these metals may cause diseases. The equilibrium of these metals is considered as preconditioning for normal immune defense and general health of our body. Hence, heavy metals are intentionally added and processed with herbal plant extracts to produce herbo-metallic/mineral drugs [28].
In-vitro cell cultures are a precious tool for toxicological assessments of various substances. Detecting the levels of cytotoxicity provides an economical method for evaluating the toxicity of compounds. In the present study, we have conducted the cytotoxicity of ZingiVir-H, a herbo-mineral drug and its components HgS and As2S3 (raw as well as various stages of detoxification) in the L6 cell line by MTT assay. Twenty-four hour exposure of the L6 cell line to varying concentrations of raw HgS and As2S3 produced a dose-dependent reduction in the fraction of viable cells. A significant reduction in the cytotoxicity was recorded for purified HgS, As2S3 and the ZingiVir-H tablets when compared to the raw HgS and As2S3. HgS, one of the ingredients used to prepare ZingiVir-H is well known for its curative effect, and the presence of sulfur in it neutralizes the toxicity and also enhances the therapeutic effect [29]. We have proved these attributes in our study and gathered promising results to back up them. The results of our study clearly pointed that ZingiVir-H did not induce any toxicity to L6 cell lines at low concentrations. Moreover, the toxicity was considerably reduced at higher concentration when compared to raw HgS and As2S3, and this may be due to the presences of purified metals used for its preparation. Based on the results obtained from our study, we can clearly establish that, by following the detoxification processes as advised in the Ayurvedic manuscripts, the toxicity will get removed. Hence, our study further brought forward the scientific support for toxicity reduction of heavy metals on its traditional detoxification steps as recommended in the classical texts of Ayurveda. Further, for ensuring the safety of ZingiVir-H drug, we have conducted the systemic toxicity studies of the drug in animals. Unambiguously, a routine administration of ZingiVir-H for 28 days did not show any signs of toxicity. Moreover, abnormalities in behavior, quantity of food and water intake, body weight and relative organ weights, hematological and serum biochemical parameters were not observed. Histopathological examination of vital organs recorded normal architecture suggesting no morphological alterations after ZingiVir-H administration. These results demonstrated that ZingiVir-H did not possess the potential to induce any toxicity in the animals. Based on the above findings of our study, the No Observed Adverse Effect Level (NOAEL) of ZingiVir-H tablets in Wistar rats following oral route administration for 28 Days was found to be 1500 mg/kg body weight/day [26].
It is reported that heavy metals induce toxicity in the human body by inducing oxidative stress through the generation of ROS or by depletion of antioxidant reserves [30]. It is well known that the overproduction of free radicals, such as ROS plays a vital role in developing many chronic diseases [31]. So, we have conducted flow cytometer assay to check the ROS production of ZingiVir-H tablets and its constituents HgS and As2S3 (raw and purified HgS and As2S3 as Ayurvedic principles). Here in this experiment, the raw HgS and As2S3 induced ROS production in L6 cell lines. But the level of ROS production considerably decreased in the purified HgS and As2S3 evidently after the detoxification processes. Surprisingly, the final compound i.e. ZingiVir-H tablets recorded a little ROS production when compared to the individual purified HgS and As2S3 as well as its 2:1 ratio. The low level of ROS production in the ZingiVir-H tablets may be due to the presence of various antioxidant phytochemicals in the ingredients of ZingiVir-H tablets. Ginger is one of the major ingredients in ZingiVir-H tablets. Researchers reported that ginger stimulates the expression of several antioxidant enzymes and reduced the generation of ROS and lipid peroxidation [32,33]. Additionally, ginger could reduce the production of ROS in human fibro sarcoma cells [34].
The higher concentrations of phenolic and flavonoid compounds, especially the presence of Eugenol in the Eugenia caryophyllus and Anathallis graveolens also possess antioxidant properties and have potent radical scavenging activity, which could react with free radicals and eventually terminate the radical chain reactions. In addition, eugenol reduces ATP generation and inhibits oxidative phosphorylation and fatty acid oxidation via down regulation of c-Myc/PGC-1β/ERRα signaling pathway thus inhibits ROS production. A graveolens also helps in the production of Glutathione, a major antioxidant that helps the removal of ROS. Glutathione is the major endogenous antioxidant produced by the cells, participating directly in the neutralization of free radicals and reactive oxygen compounds [35].
The in-vitro studies of ZingiVir-H suggest no major cytotoxicity in L6 cell line model. The raw metals used in this study recorded cytotoxicity in L6 cell line model, whereas the level of cytotoxicity was considerably reduced in the purified HgS and As2S3 as well as the ZingiVir-H. Our results are emphatic that ZingiVir-H did not induce any major toxicity to L6 cell lines and this may be due to the purified HgS and As2S3 used for its preparation. The study further facilitates the scientific support for toxicity reduction of metals based on its traditional detoxification steps recommended in the classical texts of Ayurveda. We also conducted the level of ROS production in ZingiVir-H and the raw HgS and As2S3. The phytochemicals used for the preparation of ZingiVir-H also helps in the production of a large amount of Glutathione, a major antioxidant that helps prevent the process by removing ROS. Hence, it can be inferred that the study drug, ZingiVir-H is safe and free from toxicity based on the conditions tested (cell assay).
Even though detailed in vivo studies on the reduction of toxicity during detoxification of heavy metals are available in scientific literature. To date, the scientific evidence for chemistry behind the detoxification process still lacks in the literature. So, our next aim will be studying the chemistry behind the reducing toxicity of heavy metals when it undergoes detoxifying process mentioned in the classics of Ayurveda, thus giving scientific evidence for claimed non-toxicity to the scientific society. The present study will surely be an outstanding contribution to the global Ayurvedic community and traditional medicine especially using herbo-mineral-metallic drugs. Because through this study, we have effectively answered allegations raised by western medicine regarding the safety of metal mineral/based drugs. Moreover, this will certainly encourage young researchers to work on Rasashastra (Ayurvedic pharmaceutics) areas of research for its validation and thus promoting Ayurveda to a greater height.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors give adored Pranams to “Aadhyathma Chinthalayesan”, Chinthala Ashram, Pothencode, Trivandrum, Kerala, India for his benevolence and blessings. We sincerely acknowledge Pankajakasthuri Herbal Research Foundation, Kattakada, Thiruvananthapuram, Kerala, India and Pankajakasthuri Herbals India Pvt. Ltd Poovachal, Kattakada, Thiruvananthapuram, Kerala, India for providing necessary support to conduct studies. Further, we thank all the Directors and staffs of Pankajakasthuri Herbal Research Foundation and Pankajakasthuri Herbals India Pvt. Ltd for their supports to complete this work successfully. Special thanks to Dr. Nishanth Kumar, Senior Research Associate. The authors sincerely thank The Director, CSIR-NIIST, Trivandrum, India for providing the necessary infrastructure for cell line and ROS studies. The authors are also indebted to The Director, RGCB, Trivandrum, India, for technical advice.
The authors received no financial support for the research, authorship, and/or publication of this article.
Citation: Nair JH, Sasidharan S(2021) In-vitro Cytotoxicity Assessment of ZingiVir-H Tablets, a Novel Herbo-Mineral Antiviral Drug along with Purified Mercuric Sulphide and Arsenic Trisulphide used for its Preparation, and its Corresponding Empirical Data To Reinforce The Evaluation. Drug Des. 10:191.
Received: 23-Aug-2021 Accepted: 06-Sep-2021 Published: 13-Sep-2021 , DOI: 10.35248/2169-0138.21.10.191
Copyright: © 2021 Nair JH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.