Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Mini Review - (2025)Volume 13, Issue 1
In this article, the effect of increasing temperature on the decrease of viscosity of aqueous humor is explained by analyzing the effect of temperature and the physical relationship of viscosity. First, we extract and examine the physical relations governing the viscosity of fluids, because 90% of aqueous humor is water and the rest is dissolved in it. Water such as sodium, potassium and has formed, therefore, the physical perspective on fluids is effective, so firstly, the physical relations governing the extraction fluids and the effect of temperature on the aqueous humor are investigated and finally, its relationship with the internal pressure of the eye is explained.
Intraocular pressure; Fluid temperature; Flow conditions; Pressure; Multiphase flow
Intraocular Pressure (IOP) within the physiological range is essential for ocular homeostasis. Decreased intraocular pressure can lead to pathologies such as choroidal effusion and tuberculosis. Increased intraocular pressure can cause corneal edema and glaucoma. Glaucoma is one of the most common causes of irreversible blindness worldwide [1-3]. Although several different influencing factors have been identified so far, IOP is the most important risk factor for the development of glaucoma or disease progression [4]. Therefore, most glaucoma treatments focus on regulating intraocular pressure. In healthy people and glaucoma patients, intraocular pressure has been shown to exhibit diurnal and nocturnal fluctuations [5]. Seasonal fluctuations, temperature changes, dependence on different weather conditions. The condition has been studied in relatively small cohorts in the northern hemisphere [6-12]. Several studies have found peaks in IOP values during winter and therefore when temperatures are low [13,14]. The possible effects of these fluctuations have recently been reported, such as the occurrence of optic disc hemorrhage in response to seasonal and temperature fluctuations [15]. These results indicate that these seasonal changes need to be studied in detail to better understand their possible effects on glaucoma and its progression.
Physical relationships governing viscosity
Internal friction in a liquid is called viscosity. Water and honey are two very famous examples to understand the concept of viscosity. Consider an incline. If we pour water on a sloping surface, the water will move quickly from the top of the surface to the bottom. Now, if we pour some honey on top of this surface, the honey will slowly move from the top to the bottom. Therefore, water has a greater flow rate than honey. The reason for this is that the viscosity of honey is much greater than that of water. If the viscosity of a fluid is large, the fluid will resist motion because strong intermolecular forces result in very large internal friction. Therefore, this friction prevents the fluid layers from moving easily relative to each other. On the contrary, the fluid flow with low viscosity will be very easy, because the intermolecular forces in this fluid are much smaller than the intermolecular forces in a liquid with high viscosity and as a result, the internal friction between the layers of the small fluid and the movement of these layers relative to each other. It's easier. Note that viscosity is observed not only in liquids but also in gases, but under normal conditions it will be difficult to detect.
What are the factors affecting viscosity?
Fluid temperature, flow conditions, pressure, multi-phase flow and suspended particles are factors affecting viscosity. In the following, we will explain about each of them.
Fluid temperature: Normally, the viscosity of liquids decreases rapidly with increasing temperature, but the viscosity of gases increases with increasing temperature. Therefore, liquids flow more readily as the temperature increases, while gases flow more slowly. Also, the viscosity will not change with the change in the amount of substance.
Flow conditions: When the liquid is flowing smoothly, the viscosity remains constant, but the viscosity value will change in turbulent flows.
Pressure: As the pressure increases, the viscosity of gases usually increases. Pressure has little effect on the viscosity of liquids.
Multiphase flow: The viscosity of multiphase flow is affected by the volume of each phase.
Suspended particles: Suspended substances increase viscosity.
Effect of temperature on viscosity
First, we talk about the effect of temperature on viscosity. Temperature has a great effect on viscosity. The same fluid at two different temperatures T1 and T2 Consider and assume T2 is bigger than T1. The viscosity of the desired fluid at a higher temperature will be very different from the viscosity of the same fluid at a lower temperature. Viscosity represents the molecular behavior of different samples, therefore, the temperature change during viscosity measurement gives us a lot of information about the micro structural behavior of the fluid. Viscosity increases with temperature because the particles move faster and collide with each other in a shorter time. In liquids, the intermolecular force is strong, when the temperature increases, the kinetic energy of the liquid molecules and the distance between them increases. Therefore, their intermolecular force will decrease. Hence, the internal friction and thus the viscosity is reduced. Measuring and understanding viscosity at different temperatures is necessary to formulate its various applications. Extreme changes in viscosity with temperature have a large impact on complex formulations such as motor oil, antibody-based therapeutics, hemp oil and personal care products. When cooking and using oil or butter, you will notice drastic changes in viscosity. As the temperature increases, the viscosity of butter or oil decreases and they move easily in the pan. Fluid viscosity can be calculated by dropping a sphere into the liquid and using the following equation:
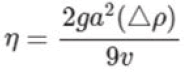
In the above relation, is the density difference between the liquid and the sphere, a is the radius of the sphere, g is the acceleration of gravity on the surface of the earth and v is the speed of the sphere.
Viscosity of gases
Gas viscosity is defined as its resistance to flow. The unit of gas viscosity in the CGS system is poise or dyne-second per square centimeter. The viscosity of gases at a temperature close to room temperature is in the traditional poise range and therefore this unit is a common unit for measuring the viscosity of gases. The dependence of the viscosity of gases will be very low on pressure and very high on temperature. The viscosity of gases is written as follows based on Sutherland's formula:
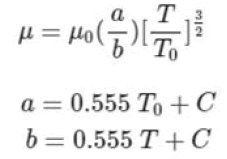
The Figure 1 below shows the comparison between the viscosity of a gas and a liquid.
Figure 1: The comparison between the viscosity of a gas and a liquid.
Experimental results on patients
In a glaucoma surgery under general anesthesia by our treatment staff, we treated the patient by heating the normal-saline serum during the operation and after the surgery, we measured the intraocular pressure by placing a warm compress in the recovery area and on the other hand, we performed a glaucoma operation in another patient who was similar to the previous patient without heating the serum and placing a warm compress during recovery and it was observed that the patient who received warm serum had a lower eye pressure compared to the initial pressure.
We measured the viscosity of aqueous humor at a temperature between 37 and 39 and using mathematical curve fitting, we estimate the temperature graph according to the viscosity of aqueous humor and the following Table 1 and Figure 2 appears.
| Temperature (°C) | Viscosity (mPa·s or cP) |
|---|---|
| 10 | 1.3059 |
| 20 | 1.0016 |
| 30 | 0.79722 |
| 50 | 0.54652 |
| 70 | 0.40355 |
| 90 | 0.31417 |
Table 1: Viscosity of aqueous fluid at various temperatures.
Figure 2: Viscosity of aqueous fluid versus temperature in Kelvin.
Considering that the aqueous humor has a very low viscosity, it is constantly settled and renewed every 3 hours and since the aqueous humor together with the vitreous keep the eye pressure constant and affect it, according to the figure above, it can be concluded that if the temperature raise the eye (for example, by heating serums, we raise the body temperature), we have reduced the viscosity of the aqueous humor and its settling time increases and vice versa and by controlling the viscosity can be controlled the pressured of eye blood.
Analysis
Blood viscosity is 2.3 to 4.1 centipoises in a normal state and at 37 degrees celsius, as the body temperature increases, blood viscosity decreases and since viscosity is inversely related to velocity, blood velocity increases, while the viscosity of aqueous humor is less than 1. And with increasing temperature, its viscosity decreases like the viscosity of blood and its speed increases. Now, if we want to check the level of gas exchange in blood vessels and aqueous humor without taking into account interstitial elements, we find that viscosity has a direct relationship with resistance, so with increasing the temperature, the resistance of the vessels decreases and according to the relation of the resistance of the vessels, which has an inverse relationship with the radius to the power of 4, then with the decrease of the resistance of the vessels, the radius of the vessel increases and this increase increases the radius of the cross section and the exchange of carbon dioxide and oxygen increases, but since the viscosity carbon dioxide 0.01480 and oxygen viscosity 0.02018, therefore, the speed of carbon dioxide transfer from the aqueous humor to the vessel is higher than the oxygen transfer speed to the aqueous humor, so the time for the aqueous humor to clear increases from the time of the aqueous humor renewal and in glaucoma, where the increase in pressure (to the extent that it can affect the aqueous humor) viscosity is increasing and exchange rate is decreasing. With this increase in temperature, we have increased exchange, which directly helps to reduce eye pressure in glaucoma patients.
In this article, the effect of increasing the temperature on the reduction of eye pressure was investigated, especially in glaucoma patients. By increasing the temperature, we have reduced the viscosity of the aqueous humor and blood, we have increased the level of gas exchange between the aqueous humor and the capillary and by increasing the exchange rate of the aqueous humor, we have cleared the aqueous humor and we increased it compared to its renewal.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ghanbari Z, Shirzad E (2025) Investigating the Effect of Temperature on the Viscosity of Aqueous Humor from the Perspective of Classical Physics and its Relationship with Intraocular Pressure. Adv Tech Biol Med. 12:458.
Received: 09-Jan-2024, Manuscript No. ATBM-24-29087; Editor assigned: 11-Jan-2024, Pre QC No. ATBM-24-29087; Reviewed: 25-Jan-2024, QC No. ATBM-24-29087; Revised: 06-Mar-2025, Manuscript No. ATBM-24-29087; Published: 13-Mar-2025 , DOI: 10.35248/2379-1764.25.13.458
Copyright: © 2024 Ghanbari Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.