Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Mini Review - (2022)Volume 13, Issue 2
Oxalate nephropathy is a devastating renal disease with poor prognosis which can often cause a gradual kidney dysfunction with a significant risk of End Stage Renal Disease (ESRD). Typically associated with chronic interstitial nephritis or fibrosis as well as acute kidney injury, oxalate nephropathy is characterized by deposition of Calcium oxalate (CaOx) crystals in kidney tubules. The gut plays a significant role in oxalate handling. Dysbiosis induced oxidative stress, systemic inflammation and lack of oxalate degrading bacteria remarkably contributes to the oxalate nephropathy. Here we propose a microbiome-centric theory of oxalate nephropathy where initial adaptive changes in gut microbiome embark chronic inflammation in later stage, leading to Chronic Kidney Disease (CKD)-related complication such as oxalate nephropathy.
Oxalate nephropathy; Calcium oxalate; Dysbiosis; Chronic inflammation
Oxalate nephropathy is a potentially devastating condition with acute renal injury thereby advancing To End Stage Renal Disease (ESRD).
Alterations in the gut microbiota mediates Calcium Oxalate (CaOx) crystal deposition in kidney tubules that induces the synthesis of inflammatory factors such as cytokines, Monocyte Chemoattractant Protein-I (MCP-I), Interleukins-6 (IL-6) and Tumor Necrosis Factor-Alpha (TNF-δ).
CaOx mediated inflammatory response is associated with cell death, leukocyte infiltration which leads to tubular atrophy and interstitial fibrosis thereby adding up to progressive oxalate nephropathy.
Histologically characterized by the deposition of calcium oxalate crystals in the kidney tubules, oxalate nephropathy is defined as a chronic condition with reduced kidney function [1]. Oxalate nephropathy is the cause of kidney disease in 1% of the consecutive native kidney biopsies and leads to tubular injury, intestinal fibrosis and CKD or Acute Kidney Injury (AKI) that necessitates renal replacement therapy in about half of the patients. It displays poor prognosis and is a rare cause of acute renal failure [2]. The cause of oxalate nephropathy has been categorized into either inherited disorder of glyoxylate mechanism leading to overproduction of oxalate by liver (primary oxalate nephropathy) or increased intestinal oxalate absorption from dietary sources (secondary oxalate nephropathy) [3].
Impaired oxalate homeostasis is a well-known occurrence in patients with ESRD, the underlying causes of which include: dietary restrictions, malabsorption, low blood concentration of calcium, magnesium and fiber that affects oxalate absorption, high uremic concentration, inflammatory diseases, use of medications, phosphate binders or antibiotics that affects the composition of gut microbiota both qualitatively and quantitatively thereby influencing the oxalate degrading ability of gut microbiota [4,5]. CaOx can induce renal inflammation by overexpressing Interleukins (IL) and activating NOD like Receptors Proteins (NLRP3)/inflammatory bodies that cause necrosis of renal tubular epithelial cells [6]. The NLRP3 inflasome sensors are involved in recognizing oxalate, uric acid and cysteine crystals in the kidneys and hence promote the formation of inflammatory factors that lead to progressive kidney damage. Studies show that mice with inhibition of NLRP3 genetically or pharmacologically are predominantly resistant to oxalate nephropathy [7]. Although, till date the effect of gut dysbiosis on pathogenesis and production of inflammatory factors in CKD has not been extensively studied, however lately, we made an attempt to defined an association between gut dysbiotic pattern in patients with chronic kidney diseases such as renal carcinomas [8].
Dysbiosis or changes in the composition and function of microbiota have been reported in numerous illnesses including CKD. Several retrospective studies of CKD and hemodialysis patients have revealed that the number of Bifidobacterium, Lactobacillaceae, Oxalobacter, Bacteroidaceae and Prevotellaceae sp. were notably low as compared to the pro inflammatory Enterobacteriaceae (especially Enterobacter, Klebsiella, and Escherichia), Enterococci, and Clostridium perfringens that were significantly high [8,9]. Several bacterial strains such as Oxalobacter spp., Bifidobacterium spp., and Lactobacillus spp., can degrade oxalate and may be capable of modulating intestinal oxalate secretion that could predispose to enteric hyperoxaluria [10]. The dysbiosis of the gut microbiota leads to chronic immune activation due to overgrowth of pathogenic bacteria that release harmful substances such as lipopolysaccharides, bacterial DNA, peptidoglycans or outer membrane proteins into the host circulation which activate intestinal mucosal immune systems thereby promoting the generation of inflammatory factors like interleukins, Tumor Necrosis Factor δ (TNF-δ) and Interferon (INF-γ), that acts as a major risk factor for progression of CKD (Figure 1) [11].
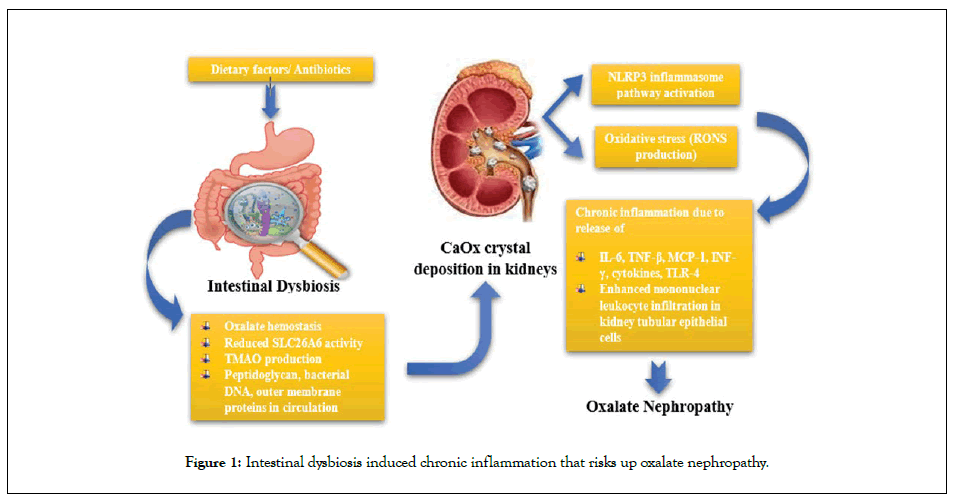
Figure 1: Intestinal dysbiosis induced chronic inflammation that risks up oxalate nephropathy.
Low abundance of oxalate degrading Oxalobacter formigenes, Bifidobacterium and Lactobacillus sp. and increase in Enterobacteriaceae has been associated with higher urinary and plasma oxalate levels (oxalate hemostasis) in patients with ESRD [12]. Nazzal and co-workers reported a case of oxalate nephropathy secondary to high dietary oxalate in a patient not colonized with O. formigenes [13]. Oxalate induces toxic responses through the activation of phospholipase A2 that results in production of several lysophospholipids and arachidonic acids which ultimately results in production of RONS (reactive oxygen and nitrogen species), mitochondrial dysfunction and changes in expression of genes that are involved in activation of caspases and apoptosis or in inhibition of calcium oxalate crystal formation. Also, the presence of significant amounts of endotoxins and expression of Toll like receptors (especially TLR4) in renal epithelial cells suggest an interaction between the high oxalate levels and activation of inflammatory responses [14]. The RONS produced by the activated NADPH oxidase, oxidises Low Density Lipoproteins (LDL) to minimally modified LDL (mm LDL) which induces the synthesis of inflammatory factors such as cytokines, Monocyte Chemoattractant Protein-I (MCP-I), Interleukins-6 (IL-6) and TNF-δ [5]. Moreover, lack of O. formigenes, oxidative stress and chronic inflammation contribute to diminished SLC26A6 mediated transcellular oxalate transfer and paracellular intestinal oxalate absorption that significantly contributes to hyperoxaluria and deposition of CaOx crystals in kidney tubules [15]. The SLC26 and SLC4 anion exchange transporters significantly increase the oxalate absorption from the intestines in a state of subclinical malabsorption which results in enhanced enteric hyperoxaluria. The deposition of CaOx crystals in the proximal renal tubules could bring about renal fibrosis along with chronic inflammation thereby elevating risks of pyelonephritis recurrence [16].
The cell model of cell crystal interaction and rat model of CaOx kidney stones experimental study conducted by Sun and his coworkers demonstrated the involvement of oxidative stress, TLR4/ NF-κB, and NLRP3 inflammasome pathways in renal inflammatory responses induced by CaOx crystals [17,18]. Stimulation of TGF-β (tumor growth factor-β) by the NLRP3 inflammasome pathway (the similar pathway as that noted in crystal induced inflammation in gout and arteriosclerosis) has been amalgamated with kidney fibrosis and progressive loss of kidney function in animal models of hyperoxaluria [10]. An acute case of oxalate nephropathy was reported in a 65-year old woman temporarily associated with consumption of high oxalate green vegetable smoothie diet with gastric bypass surgery and recent prolonged use of antibiotics as a predisposing factor. Kidney biopsies revealed presence of CaOx crystals in the cytoplasm of tubular epithelial cells consequently leading to acute tubular epithelial injury and interstitial fibrosis with a mild chronic inflammatory infiltrate of mononuclear leukocytes [19]. A systemic review of the patients presented with secondary oxalate nephropathy was conducted by Lumlertgul and co-workers. According to the investigations of kidney biopsies, about 71% of the patients presented acute tubular injury, 69% presented tubular damage and atrophy while 72% of these also reported interstitial mononuclear cell infiltration. Of these, more than 50% patients’ required renal replacement therapy and about 33% died [20].
Intestinal dysbiosis leads to enhanced production of uremic toxins such as indoxyl sulfate, p-cresyl sulfate and trimethylamine-N-oxide [21]. Recently, it has been described that Proteus mirabilis, a gramnegative intestinal bacterium which is usually found in a considerable amount in a dysbiotic pattern, produces Trimethyamine N-Oxide (TMAO) that leads to increased expression of pro-inflammatory cytokines and leukocyte adhesion in addition to activation of endothelial MAPK (Mitogen Activated Protein Kinase) pathway [14]. Lately, Dong and his colleagues also hypothesised that TMAO aggravates Calcium oxalate (CaOx) crystal deposition in the kidneys via CaOx-mediated cell death. According to the investigation, in C57B1/6 mice TMAO intensified high oxalate diet induced kidney injury by triggering Protein Kinase R-Like Endoplasmic Reticulum Kinase (PERK/ROS) pathway that enhances, autophagy, apoptosis and inflammation that facilitates CaOx crystal deposition in renal tubular cells thereby leading to oxalate nephropathy [22]. Lu and co-workers recently explained that IS increased Arachidonate 12-Lipoxygenase (ALOX12) expression and endogenous ligand 12(S)-Hydroxyeicosatetraenoic Acid (12(S)-HETE) to activate Transient Receptor Potential Vanilloid 1 (TRPV1) channel that plays a role in in renal tubular cell damage and hyperoxaluria induced renal inflammation [23].
The review seems to define a significant co-relation between gut microbiota and oxalate nephropathy. Alterations in the gut microbiome induced by medicines or dietary factors leads to activation of systemic inflammation a pathway that plays a significant role in progression of oxalate nephropathy. Commenced in the gut, the oxalate prompted molecular mechanisms concomitantly result in activation of oxidative stress, chronic inflammation and hence oxalate nephropathy. Therefore, a better apprehension of the crosstalk between the gut microbiota, inflammation and kidney is of great need that can facilitate the evolution of novel, revolutionary strategies for the prevention and treatment chronic kidney diseases such as oxalate nephropathy.
This article does not contain any studies with human participants or live animals performed by any of the authors.
The authors declare that they do not have any conflict of interest among themselves or with the host institution.
The authors are thankful to Department of Biotechnology, and Bioinformatics Centre, Himachal Pradesh University, Shimla (India) for providing resources to the authors as well as to the Department of Biotechnology, Ministry of Science & Technology, Government of India for providing financial support to the parent department.
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
Citation: Gupta S, Kanwar SS (2022) Intestinal Dysbiosis Induced Chronic Inflammation That Risks up Oxalate Nephropathy. J Cell Sci Therapy. 13:342.
Received: 21-Jan-2022, Manuscript No. JCEST-22-15535; Editor assigned: 25-Jan-2022, Pre QC No. JCEST-22-15535 (PQ); Reviewed: 04-Feb-2022, QC No. JCEST-22-15535; Revised: 11-Feb-2022, Manuscript No. JCEST-22-15535 (R); Published: 18-Mar-2022 , DOI: 10.35248/2157-7013-22.13.342
Copyright: © 2022 Gupta S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.