Journal of Alcoholism & Drug Dependence
Open Access
ISSN: 2329-6488
ISSN: 2329-6488
Research Article - (2022)Volume 10, Issue 6
Background: The aim of this investigation was to study the effect of N-phenyl-N-(1-cyclopropylethyl) nicotinamide and its possible metabolites-hydrochlorides of N-(1-cyclopropylethyl) amine and N-phenyl-N-(1-cyclopropylethyl) amine on the ethanol oxidation enzymes activity in vitro, as well as the kinetics of their interaction.
Methods: The studies were performed using alcohol dehydrogenase and aldehyde dehydrogenase is enzyme forms of rat liver subcellular fractions, which were obtained by differential centrifugation. Enzyme activity was determined spectrophotometrically. The kinetic nature of enzymes interaction with nicotinamide derivative was investigated in the concentration range of 25 μM-75 μM. The obtained results were processed by the Line weaver-Burk method.
Results: Studies have shown that N-phenyl-N-(1-cyclopropylethyl) nicotinamide reduced the rate of the reverse alcohol dehydrogenase reaction in the presence of NADH by 46% with an inhibition constant of 53 μM. Soluble mitochondrial aldehyde dehydrogenase activity was inhibited by 50% with an inhibition constant of 108 μM. The kinetic nature of the substituted nicotinamide interaction with enzymes at saturating concentrations of reaction cofactors NADH and NAD+ was quite complex. Allosteric effects can play a significant role in enzymatic activity. Possible metabolites of the test compound, hydrochlorides of N-(1-cyclopropylethyl) amine and N-phenyl-N-(1- cyclopropylethyl) amine, didn’t significantly alter the ethanol metabolic enzymes activity.
Conclusions: A new inhibitor of the rate of the reverse alcohol dehydrogenase reaction and the activity of the soluble mitochondrial is enzyme form of aldehyde dehydrogenases, which alter the ethanol metabolism and lead to the acetaldehyde accumulation in the body, has been identified. N-phenyl-N-(1-cyclopropylethyl) nicotinamide can be used as a potential anti-alcohol drug with sensitizing action.
Alcohol dehydrogenase; Aldehyde dehydrogenase; Disulfiram alcohol reaction; Inhibitor; Sensitizing drugs
One of the methods of chronic alcoholism treatment and ant relapse therapy is the use of sensitizing agents that significantly increase the body's sensitivity to alcohol. The main way of body ethanol metabolism is its transformation with the participation of Alcohol Dehydrogenase (ADH, EC 1.1.1.1), which catalyzes the alcohols oxidation (direct reaction) and the aldehydes reduction (reverse reaction) in the presence of cofactors NAD+ and NADH, respectively. Further acetaldehyde oxidation to acetic acid occurs with the participation of Aldehyde Dehydrogenase (ALDH, EC 1.2.1.3) in the presence of reaction cofactor NAD+ [1-3]. Inhibition of this enzyme activity leads to an acetaldehyde concentrations increase and its accumulation in an organism [4,5].
As a result of acetaldehyde intoxication, severe disorders of the body (so-called Disulfiram-Alcohol Reaction (DER)) occur, which encourages patients to reduce or stop drinking alcohol. In the body, disulfiram (Tetra Ethylthiuram Disulfide (TETD) or Antabuse) produces such unpleasant side effects as fast heartbeat, chest pain, nausea, dizziness, flushing, and thirst when combined with alcohol. However, long-term use of this drug can be accompanied by the development of serious complications and even death [6,7].
Therefore, a promising area of treatment is the new effective drugs investigation, as well as the creation of combined drugs that have greater specificity and lower toxicity. For example, combined drug for the treatment of alcohol dependence Lidevine contains disulfiram, nicotinamide and adenine. Such composition reduces the disulfiram toxicity and the risk of side effects, provides a pronounced manifestation of alcohol disulfiram reaction at low drug and alcohol doses, improves the treatment outcome, and provides prevention of alcoholic neuropathy and hypovitaminosis [8].
A similar disulfiram alcohol reaction is caused by micotoxin coprine isolated from the mushrooms coprinopsis atramentarius and identified as N-(1-hydroxycyclopropyl)-L-glutamine. Aminocyclopropanol is an active metabolite of this compound.
The main aim of this investigation was to study the effect of Nphenyl- N-(1-cyclopropylethyl) nicotinamide and its possible metabolites: hydrochlorides of N-(1-cyclopropylethyl) amine and N-phenyl-N-(1-cyclopropylethyl) amine on the activity of ethanol metabolic enzymes and kinetic nature of their interaction in vitro.
The following reagents were used: sodium deoxycholate, rotenone, NAD+ and NADH (Sigma, USA); human serum albumin (Reanal, Hungary); Coomassie Brilliant blue G-250 ("Serva", USA). The other reagents were analytical grade. The studied compounds were synthesized at the Institute of Bioorganic Chemistry and Stereochemistry of the National Academy of Sciences of Ukraine, Kyiv.
Subcellular fractions of the liver of outbred white rats were obtained by differential centrifugation at 4⁰C. The protein concentration of the cytoplasmic fraction was determined by direct spectrophotometry [9], other fractions by the Bradford method [10]. The rates of forward and reverse ADH reactions and the activity of different ALDH isoenzyme forms were determined spectrophotometrically at a wavelength of 340 nm and expressed in nmol NADH/(mg protein∙min) [11]. The reaction mixture contained: 1) for ADH: 0.1 M Na-phosphate buffer (pH=7.3), 0.040 M KCI, 500 μg protein, for direct reaction - 1 mM NAD+ and 1-5 mM ethanol, for reverse reaction - 0.5 mM NADH and 0.25-1 mM acetaldehyde; 2) for ALDH: 50 mM Na-pyrophosphate buffer (pH=8.8); 500 μg of protein; 0.5 mM NAD+; 0.0125-5 mM acetaldehyde, 1 μM rotenone for mitochondrial fraction (NADH oxidase inhibitor). The total volume of the sample was 3 mL. Samples were preincubated for 5 minutes at a temperature of 37°C. The reaction was started by the substrate adding. Measurements were performed for 5 min on the spectrophotometer "Beckman DU-6" (USA).
Under in vitro conditions, the kinetics of the enzymes interaction with potential modifiers was studied by adding of substances solutions at concentrations of 25–75 μM to the analyzed mixture after preliminary incubation for 5 min. Nphenyl- N-(1-cyclopropylethyl) nicotinamide was dissolved in ethanol with a volume fraction of 96%; hydrochlorides of N-(1- cyclopropylethyl) amine and N-phenyl-N-(1-cyclopropylethyl) amine were dissolved in water. The obtained data was presented in the coordinates of double reciprocal values by the Lineweaver- Burk method.
N-phenyl-N-(1-cyclopropylethyl) nicotinamide (I) contains cyclopropyl residue - an active component from the mushrooms coprinopsis atramentarius, which has a sensitizing effect. Nicotinamide residue is an integral part of different biologically active substances, which play an important role in biochemical processes in the body. In particular, NAD+ and NADH are necessary for the functioning of enzymes of the ethanoloxidizing system and can reduce the toxic effect of drugs. The effect of substituted nicotinamide and its possible metabolites hydrochlorides of N-(1-cyclopropylethyl) amine (II) and Nphenyl- N-(1-cyclopropylethyl) amine (III) - on the enzymes activity of the ethanol-metabolizing system are shown in (Table 1).
| Code of compounds | ADH activity | ALDH isoenzymes activity | |||
|---|---|---|---|---|---|
| Forward reaction | Reverse reaction | Soluble mitochondrial | Membrane mitochondrial | Membrane microsomal | |
| Control | 6.32 ± 0.96 | 5.83 ± 0.66 | 9.61 ± 0.67 | 14.88 ± 1.98 | 7.26 ± 0.97 |
| І | 5.12 ± 0.89 | 3.17 ± 0.45* | 4.83 ± 0.81* | 13.86 ± 1.83 | 6.29 ± 1.45 |
| ІІ | 5.69 ± 0.89 | 6.12 ± 0.92 | 8.13 ± 0.67 | 16.67 ± 2.14 | 7.05 ± 0.78 |
| ІІІ | 6.64 ± 1.18 | 5.66 ± 1.05 | 9.45 ± 1.03 | 15.72 ± 1.14 | 8.18 ± 1.13 |
Note: * -95% СI
Table 1: The effect of N-phenyl-N-(1- cyclopropylethyl)nicotinamide (I),N-(1-cyclopropylethyl)amine (II) and N-phenyl-N-(1- cyclopropylethyl)amine (III)on the activity of ethanol metabolic enzymes (nmol NADH/(mg protein min), M ± m, n=6)
Studies have shown that in vitro condition N-phenyl-N-(1- cyclopropylethyl) nicotinamide (I) inhibited the activity of ADH, which catalyzes the reverse reaction of the acetaldehyde reduction to ethanol in the presence of NADH by 46%.
In the rat liver, ALDH is represented by three are enzyme forms: soluble mitochondrial form, membrane mitochondrial and membrane microsomal forms. The soluble mitochondrial form plays a major role in the acetaldehyde oxidation in physiological conditions. Membrane-bound forms are activated during chronic alcoholism. Studies have shown that cyclopropylethylcontaining nicotinamide reduced only soluble mitochondrial ALDH activity by 50%.
Possible metabolites of substituted nicotinamide hydrochlorides of N-(1-cyclopropylethyl) amine (II) and N-phenyl-N-(1- cyclopropylethyl) amine (III) didn’t significantly alter the activity of ethanol metabolic enzymes. Thus, the inhibitory ability was shown by N-phenyl-N-(1-cyclopropylethyl) nicotinamide on the whole, while micotoxin coprine is inactive in vitro, and its metabolite 1-aminocyclopropanol inhibits soluble ALDH in vivo.
From the point of view of enzymatic kinetics, reactions involving ADH and ALDH are two-substrate (alcohol (aldehyde) and NAD+ (NADH)) and occur by an ordered Bi-Bi mechanism. The addition of NAD+ (NADH) changes the conformation of the enzyme and promotes further binding of alcohol or aldehyde . Inhibition of the reverse ADH reaction rate and soluble mitochondrial ALDH activity leads to the accumulation of acetaldehyde in the body. N-phenyl-N-(1-cyclopropylethyl) nicotinamide reversibly interacted with both enzymes, since stabilization of enzyme activity was observed during their pre incubation.
The effect of the inhibitor on the reverse ADH reaction rate was determined at saturating NADH concentrations and various acetaldehyde concentrations (Figure 1a). The obtained data were presented as double reciprocal plot by the Line weaver-Burk method . At low acetaldehyde concentrations, the amide acted as an activator. With an increase in the acetaldehyde concentration, inhibition of the rate of the reverse ADH reaction was observed. Mainly, such complex character of inhibition is typical for multi substrate reactions. The addition of the inhibitor alters the conformation of the enzyme molecule in such a way that the interaction of the substrate with the active site of the enzyme is disrupted and the rate of the enzymatic reaction is reduced.
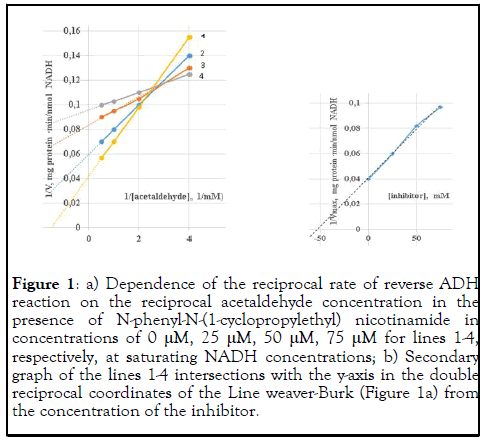
Figure 1: a) Dependence of the reciprocal rate of reverse ADH reaction on the reciprocal acetaldehyde concentration in the presence of N-phenyl-N-(1-cyclopropylethyl) nicotinamide in concentrations of 0 μM, 25 μM, 50 μM, 75 μM for lines 1-4, respectively, at saturating NADH concentrations; b) Secondary graph of the lines 1-4 intersections with the y-axis in the double reciprocal coordinates of the Line weaver-Burk (Figure 1a) from the concentration of the inhibitor.
According to the secondary graph of the dependence of the lines 1-4 intersections with the y-axis (Figure 1a) in the coordinates of Line weaver-Burk from the concentration of the inhibitor, the inhibition constant was 53 μM (Figure 1b).
For soluble mitochondrial ALDH, the nature of inhibition against acetaldehyde at saturating NAD+ concentrations varied from uncompetitive (line 2 and 3 at amide concentrations up to 50 μM) to mixed (line 4) (Figure 2a).
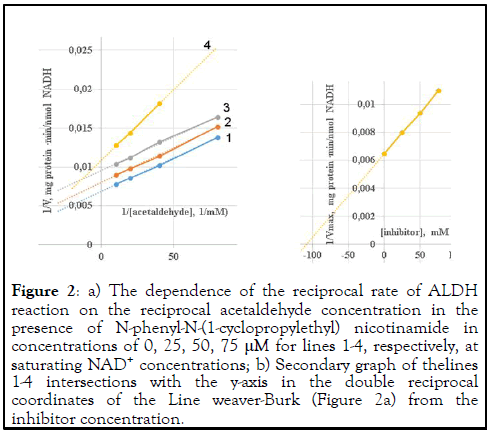
Figure 2: a) The dependence of the reciprocal rate of ALDH reaction on the reciprocal acetaldehyde concentration in the presence of N-phenyl-N-(1-cyclopropylethyl) nicotinamide in concentrations of 0, 25, 50, 75 μM for lines 1-4, respectively, at saturating NAD+ concentrations; b) Secondary graph of thelines 1-4 intersections with the y-axis in the double reciprocal coordinates of the Line weaver-Burk (Figure 2a) from the inhibitor concentration.
At low concentrations, the inhibitor can only bind with enzymesubstrate complex and inactivate its. With increasing concentrations, interaction with allosteric centers of the enzyme is possible, which makes its configuration unsuitable for efficient catalysis.
According to the secondary graph of the dependence of the lines 1-4 intersections with the y-axis in the Line weaver-Burk coordinates on the concentration of the inhibitor (Figure 2b), the inhibition constant was 108 μM.
Studies have shown that N-phenyl-N-(1-cyclopropylethyl) nicotinamide is able to inhibit the rate of the reverse ADH reaction, which reduces acetaldehyde to ethanol in the presence of NADH, by 46%, and the activity of soluble mitochondrial ALDH by 50%. Thus, the simultaneous inhibition of this ethanol metabolism enzymes activity leads to the acetaldehyde concentration increase. Possible metabolites of the studied compound, hydrochlorides of N-(1-cyclopropylethyl) amine and N-phenyl-N-(1-cyclopropylethyl) amine, don’t significantly change the activity of ethanol metabolism enzymes in vitro.
Compared with disulfiram, the advantage of N-phenyl-N-(1- cyclopropylethyl) nicotinamide is its reversible effect on enzymes activity. The kinetics of its interaction with enzymes is quite complex, since ADH and ALDH consist of several subunits. Allosteric effects can play a significant role in the activity of enzymes. To assess the possibility of N-phenyl-N-(1- cyclopropylethyl) nicotinamide using in complex ant alcoholic therapy as a sensitizing agent, further study of its action in vivo on a model of chronic alcoholism is necessary.
I would like to thank the staffs of the Institute of Bioorganic Chemistry and Petro chemistry of the National Academy of Sciences of Ukraine, Kyiv, who synthesized the compounds studied in this work.
The author received no financial support for the research, authorship, and/or publication of this article.
Citation: Kyslova O (2022) Influence of Nicotinamide Derivative and its Possible Metabolites On the Ethanol Metabolic Enzymes Activity. J Alcohol Drug Depend. 10:366.
Received: 26-Apr-2022, Manuscript No. JALDD-22-17173; Editor assigned: 29-Apr-2022, Pre QC No. JALDD-22-17173(PQ); Reviewed: 13-May-2022, QC No. JALDD-22-17173; Revised: 27-Jun-2022, Manuscript No. JALDD-22-17173(R); Published: 04-Jul-2022 , DOI: DOI: 10.35248/2329-6488.22.10.373
Copyright: © 2022 Kyslova O. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.