Drug Designing: Open Access
Open Access
ISSN: 2169-0138
ISSN: 2169-0138
Market Analysis - (2020)
The global nanomedicine market size was estimated at USD 138.8 billion in 2016. Technological advancements coupled with relevant applications in early disease diagnosis, preventive intervention, and prophylaxis of chronic as well as acute disorders is expected to bolster growth in this market. Nanotechnology involves the miniaturization of larger structures and chemicals at nanometric scale which has significantly revolutionized drug administration, thus influencing adoption of the technology through to 2025.
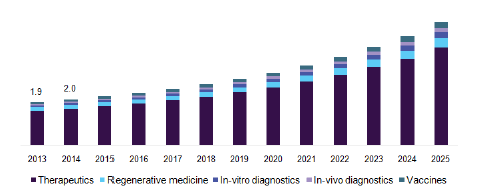
North America dominated the nanomedicine market with revenue share of over 42% owing to presence of increasingly growing partnerships between enterprises operating herein and nanomedicine startup organizations. Furthermore, support from the government entities coupled with higher R&D spending is attributive for largest share of region in the industrial space.
However, Asia Pacific is expected to witness lucrative growth through to 2025 as a result of rise in number of research grants and increase in demand for prophylaxis of lifethreatening diseases. Moreover, rise in the number of venture capital investors from developing economies of this region and increasing international research collaborations are anticipated to propel growth in nanotechnology-based healthcare industry.
Major players operating in this market are Combimatrix Corp, Ablynx NV, Abraxis Bioscience Inc., Celgene Corporation, Teva Pharmaceutical Industries Ltd, Arrowhead Research, GE Healthcare, Merck & Co. Inc., Pfizer Inc., and Nanosphere, Inc. These players are involved in gaining the U.S. FDA approvals in order to enhance the market presence.
For instance, in February 2017, company received approval from European Commission for its REVLIMID (lenalidomide) as monotherapy for treatment of patients with adult patients with multiple myeloma. This approval allows company to distribute its product in European countries, thereby enhancing company’s presence in Europe market.
Regional Outlook (Revenue, USD Billion; 2013 - 2025)
• North America
• U.S
• Canada
• Europe
• Germany
• UK
• Asia Pacific
• Japan
• China
Ali Rea
E-mail: ali@fiimas.org
USA
Published: 29-Dec-2020
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.