Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2023)Volume 12, Issue 6
Various therapies have been utilized to increase penile length and girth. P-Long evaluates a novel combination therapy using Platelet-Rich Plasma (PRP), penile traction for lengthening, vacuum erection device for girth enhancement, and nitric oxide precursor supplements to augment penile vascular smooth muscle function. A prospective, non-randomized study was designed to evaluate the efficacy of combination therapy in healthy men aged 20-55 with baseline normal erectile function and no penile pathology. Monthly penile length and girth measurements were obtained using at-home photography of an erect penis with maximal tumescence. PRP was administered in the right and left corpora under ultrasound guidance once a month for six months. Subjective erectile function was assessed with a 5-point Likert Scale. Subjects underwent twice daily penile traction for 20 minutes and suction for 12 minutes. Oral nitric oxide boosting supplementation consisted of 3 gm L-Citrulline and 1 gm beet extract. Mean age was 32 and mean baseline erect penile length was 5.70 and girth was 5.25. Erect penis length was 0.805 inch greater after six months from baseline (N=29, paired t test t=13.641 p value=0.000). Circumference was 0.469 inch greater (N=29, paired t test t=13.498 p value=0.000). Using a Likert scale, all participants reported better erectile function. No adverse events were reported. Results from a pilot study using a novel combination therapy (P-Long Protocol) in healthy men demonstrated increases in penile length, girth, and subjective erectile function improvement compared to baseline with no adverse effects.
Platelet-Rich Plasma (PRP); P-Long Protocol; Penile traction; Likert scale
Various therapies have been utilized to increase penile length and girth, with some exhibiting significant adverse event profiles, including fillers, fat transfers, suspensory ligament ligation, and silicon augmentation. Currently, there are no successful published protocols for penile growth that exclude surgery or synthetic substances.
Penile size enhancement interventions are controversial [1]. Personal aesthetic preferences lead men to seek penile augmentation [2,3]. Unfortunately, patient interest in penile enhancement techniques has not led to significantly more research and development [2,4]. The scientific literature describes suboptimal results and complications like penile deformity, Erectile Dysfunction (ED), sensory loss, and infection [4-6]. Because of an unrealistic concept of the ideal penile size, subjects tend to consider their size insufficient [7].
Penile enhancement has been attempted with several technologies, including traction devices, penile extenders, tissue fillers, surgical ligation of the accessory ligament, and silicone implants, among others. Two studies examined Penile Traction Therapy (PTT) in a total of 77 men. [8,9]. Both studies reported small but statistically significant (p<0.05) increases in baseline flaccid and stretched penile length [9].
Seven studies assessed the use of tissue fillers injected between the Dartos and Buck's fascia for girth improvement, including autologous fat [10], polylactic acid and/or hyaluronic acid [11-13], and Poly-Methyl-Meth-Acrylate (PMMA) microspheres [14-16]. Overall, all studies reported statistically significant (p<0.05) changes in penile girths, with ranging between 1.5 and 4 cm. There was no long-term follow-up or increase in erect length or penile function.
Five studies investigated suspensory ligament ligation, [17-21] which improves flaccid penile length but not penile girth or erect length.
Recently, the US Food and Drug Administration (FDA) registered an implantable silicone device for penile cosmetic enhancement: Penuma (International Medical Devices, Beverly Hills, CA, USA). A trained urologist inserts the implant over the tunica albuginea of the penis. Measurement of 400 patients document an increase in the midshaft circumference from 8.5 cm to 13.4 cm (56.7% increase; p<0.001). However, serious complications are possible, and recently there was a published series of Penumas being explanted for a variety of reasons [22,23].
Our team sought to evaluate a novel combination therapy to enhance penile characteristics using Platelet-Rich Plasma (PRP) as a source of autologous growth factors, penile traction for lengthening, vacuum erection device for girth enhancement, and nitric oxide precursor supplements to augment penile vascular smooth muscle function. The primary aim of the present study was to identify a safe and effective protocol to increase the length and circumference of the erect penis of a man with normal erectile function.
A prospective, non-randomized study was designed to evaluate the efficacy of combination therapy in healthy men aged 20-55 with baseline normal erectile function and no penile pathology. Exclusion criteria included erectile dysfunction, Peyronie’s Disease, any previous procedure on penis, and significant medical comorbidity. Penile length and girth measurements were obtained using at-home photography of an erect penis with maximal tumescence and a standardized measuring device for length and mid-shaft girth. Measurements were obtained at baseline and monthly intervals for 6 months. PRP was administered in the right and left corpora under ultrasound guidance once a month for six months using PureSpin double spin system (60 cc blood drawn). Centrifuge technical data show an average platelet recovery of 84% and an average concentration of 6.6 times that of the whole blood sample. Using this data, the injection of 10 cc of PRP, assuming a starting platelet count of 250 K/microliter, would contain approximately 1.65 billion platelets. Subjective erectile function was assessed with the question, “How would you compare your current erections with the way your erections were before starting the P-Long Study.” For the response, we used a 5-point Likert Scale (-2 much worse, -1 worse, 0 no change, 1 better, 2 much better).
RestoreX traction device was utilized for 20 minutes, twice daily, and a Dr Joel Kaplan vacuum erection device was applied with 5-10 mmHg pressure × 12 minutes (1 min × 2, 5 min × 2). Oral nitric oxide boosting supplementation was with AFFIRM, which consists of 3 gm L-Citrulline, 45 mg Redbeet root extract 4:1, 65 mg Muira Puama bark extract 4:1, and 35 mg Panax Ginseng root extract 4:1.
Traction protocol for length enhancement
The P-Long Protocol utilized the RestoreX, a traction device developed in cooperation with the Mayo Clinic and licensed to PathRight Medical (Plymouth, Minnesota, USA). Results from a randomized control trial of men with Peyronie’s Disease treated with the RestoreX device for three months demonstrated statistically significant improvement in penile length, curvature and erectile function compared with controls in 30 to 90 minutes/day [24]. Men in the P-Long Study were instructed to use the RestoreX device for 20 minutes twice daily in the straight position.
Suction protocol for girth enhancement
We instructed patients to apply the hand vacuum pump (Dr Joel Kaplan Inc Metal Hand Pump System San Diego, CA USA) to achieve a satisfactory erection with a recommended pressure between 5 and 10-mm Hg. Subjects maintained suction with full tumescence for one minute and then the vacuum was released. Vacuum suction was reapplied for 1 minute and then released and then reapplied for 5 minutes then released and then reapplied for 5 minutes and then released. This routine was performed twice a day.
Platelet rich plasma
The P-Long Protocol Utilizes the PurePRP® PS60 pure kit. The PurePRP® AB60 is a dual-spin processing technique. During the first spin, the blood product is placed in the Platinum Series Centrifuge (Emcyte Corporation, Fort Myers, FL) at the setting of PurePRP® SP SPIN 1 and this is set to 1.5 minutes at 3.8 × 103 revolutions per minute (rpm). After this cycle, the platelet plasma suspension (PPS) is then removed and placed in a concentrating accessory device before being placed back into the Platinum Series Centrifuge device for the second spin cycle, which is then set to 5.0 minutes at 3.8 × 103 rpm. After this, the platelet buffy coat leukocyte layer is separated from the rest of the plasma. The remainder of the solution is then manually shaken in the bottle prior to placement in the final syringe. One laboratory study of two samples demonstrated a mean platelet increase of 5.2 (± 0.3) fold with PurePRP® AB60 Pure [25]. Third-party research conducted at Biosciences Research Associates (BSR) by Mandle, revealed the Purespin PRO 60 yielded at 6.7 fold concentration for a total platelet count of between 1 and 1.163 billion platelets ± 450 million.
After the preparation of the injection, patients were placed in the supine position. A total of 5 cc was infused in each corpora cavernosum over 2 minutes. The whole procedure was performed under sterile conditions without anesthesia. Ultrasound guidance confirmed the proper placement of the needle in the mid-corpora. Following administration, manual compression of the base of the penis was performed for five minutes, and then a dressing was placed around the penile shaft. All PRP injections were performed by a single provider.
Nitric oxide boosting nutritional supplement
A nutritional supplement (AFFIRM from AFFIRM Science) consisting of L-Citrulline 750 mg, beet root extract 4:1 45 mg, Muira Puama extract 4:1 65 mg, and Panax ginseng root extract 4:1 35 mg was taken 2 tablets in the morning and 2 tablets in the evening.
Statistical analysis
Statistical analyses and data processing were performed using Statistical Package for the Social Sciences (SPSS) version 26. The statistical software originally was released in 1968 for mainframe computers and has been an International Business Machines (IBM) product since acquired in 2007. (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
The mean age of participants was 32 with a range of 25 to 52. The average SHIM score at baseline was 24 with a median score of 25. The patients were otherwise healthy. The average baseline penile length was 5.758 inches, and the average baseline penile girth was 5.247 inches [26,27].
Results both length and circumference increases were statistically significant at α=0.05. Erect penis length was 0.805 inch greater after six months from baseline (N=29, paired t test t=13.641 p value=0.000). Circumference was 0.469 inch greater (N=29, paired t test t=13.498 p value=0.000). Dividing by six months figures to 0.13 inch and 0.078-inch increases per month, respectively (Figure 1).
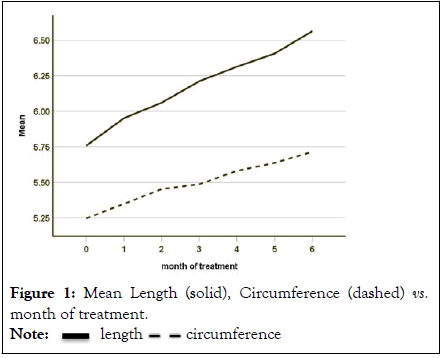
Figure 1: Mean Length (solid), Circumference (dashed) vs. month of treatment.
Note:  length
length  circumference
circumference
As determined by first order linear regression models, the continuous increases in the mean over time (Figure 1) for was 0.128 inch per month for length and 0.075 inch per month for girth, both statistically significant at α=0.05 (r2=0.068 F=11.738 p value=0.001 and r2=0.259 F=14.719 p value=0.000, respectively).
Descriptive statistics for length and circumference reveal the mean increased successively for each month (Figures 2 and 3).
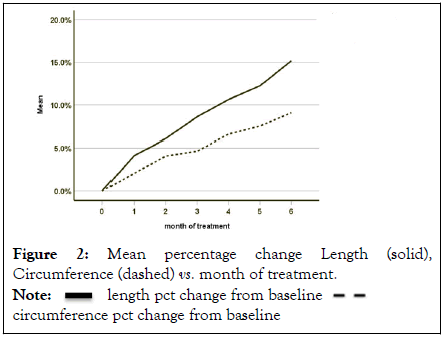
Figure 2: Mean percentage change Length (solid), Circumference (dashed) vs. month of treatment.
Note:  length pct change from
length pct change from  baseline circumference pct change from baseline
baseline circumference pct change from baseline
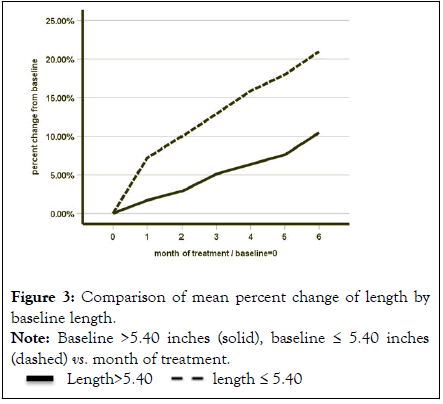
Figure 3: Comparison of mean percent change of length by
baseline length.
Note: Baseline >5.40 inches (solid), baseline ≤ 5.40 inches (dashed) vs. month of treatment.
 Length>5.40
Length>5.40  length ≤ 5.40
length ≤ 5.40
Percentage growth stated as percent increase by patient, the mean increase from baseline for length was 15.2%; for circumference was 9.1% Figure 2. Descriptive statistics show the means increase successively for each month. Dividing by six months figures to 2.53% and 1.51% increases per month, respectively.
Partitioning into two groups, those with baseline length less than or equal to 5.40 inches, reveals a difference in percentage increase from baseline Figure 3. The group ≤ 5.40 increase at 6 months is 21.0%, twice the 10.4% of the group >5.40 inches. The difference is statistically significant (N=29, t test unequal variances t=-4.035 p value=0.001). Using a Likert questionnaire, subjects were asked after the study whether their function was the same, better, much better, worse, or much worse. All 30 participants in the study responded that their function was better. No participant claimed no change or worse.
There were no side effects, short or long-term, reported by participants. No incidence of ecchymosis, hematoma, pain or long-term side effects were reported (Tables 1 and 2).
| L0-L6 | |||||||
|---|---|---|---|---|---|---|---|
| Nmonth | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| N | 29 | 29 | 29 | 29 | 29 | 29 | 29 |
| Mean | 5.758 | 5.952 | 6.06 | 6.212 | 6.314 | 6.406 | 6.564 |
| Median | 5.75 | 5.75 | 6 | 6.125 | 6.25 | 6.25 | 6.5 |
| Minimum | 2.813 | 3.875 | 3.75 | 3.5 | 3.75 | 3.75 | 4 |
| Maximum | 8.125 | 8.25 | 8.25 | 8.25 | 8.25 | 8.5 | 8.75 |
| Range | 5.313 | 4.375 | 4.5 | 4.75 | 4.5 | 4.75 | 4.75 |
| Std.dev. | 1.153 | 1.055 | 1.054 | 1.084 | 1.058 | 1.065 | 1.06 |
Table 1: Descriptive statistics for Length (L0-L6).
| G0-G6 | |||||||
|---|---|---|---|---|---|---|---|
| Nmonth | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| N | 29 | 29 | 29 | 29 | 29 | 29 | 29 |
| Mean | 5.247 | 5.349 | 5.453 | 5.487 | 5.582 | 5.637 | 5.716 |
| Median | 5.125 | 5.25 | 5.3 | 5.375 | 5.5 | 5.5 | 5.75 |
| Minimum | 4.25 | 4.375 | 4.375 | 4.4 | 4.5 | 4.5 | 4.75 |
| Maximum | 7 | 7 | 7.4 | 7.3 | 7.25 | 7.3 | 7.5 |
| Range | 2.75 | 2.625 | 3.025 | 2.9 | 2.75 | 2.8 | 2.75 |
| Std. dev. | 0.564 | 0.552 | 0.58 | 0.57 | 0.534 | 0.591 | 0.573 |
Table 2: Descriptive statistics for Circumference (Girth, G0-G6).
Results from a pilot study using a novel combination therapy (PLong Protocol) in men with no erectile dysfunction demonstrated increased penile length, girth, and subjective rigidity improvement compared to baseline with no adverse effects. These results are not unexpected since penile traction with the RestoreX in men with Peyronie's Disease has increased penile length by 0.71 inches, and PRP penile injection has been shown to improve erectile function. However, this is the first study documenting that combination therapy can simultaneously increase length, girth and function. This study is a multi-variable analysis with no control group, so it is uncertain which of the four interventions the cause of the increase are in penile size, or if the four are synergistic. In addition, at home measurement and photography is not a standard way to measure penile length and girth for the purposes of research. External validation of these results is warranted.
The benefit of enhancement with the P-Long Protocol is that it does not require synthetic injectables, surgery, or foreign bodies and does not preclude additional augmentation procedures. The current study followed men for six months, but the slope of the growth curve indicates possible continued growth with additional treatments.
JB, SL and CR designed the research study. JB performed the research, JB and SL analyzed the data. All authors read and approved the final manuscript.
This study was approved by the Integreview IRB on October 25, 2019 as a non-significant risk. The informed consent document, package label and device information was reviewed and approved.
Dean Barron performed the statistical analysis. Rena Malik MD assisted with patient recruitment.
1. This research was funded by The Cellular Medicine Association.
JB is CEO of AFFIRM Science and P-Long Inc. CR is CEO of The Cellular Medicine Association. SL has nothing to declare.
[CrossRef]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Brandeis J (2023) Increasing Penile Length and Girth in Healthy Men Using a Novel Protocol: The P-Long Study. Andrology. 12:302.
Received: 30-Jun-2023, Manuscript No. ANO-23-25339; Editor assigned: 03-Jul-2023, Pre QC No. ANO-23-25339 (PQ); Reviewed: 17-Jul-2023, QC No. ANO-23-25339; Revised: 24-Jul-2023, Manuscript No. ANO-23-25339 (R); Published: 31-Jul-2023 , DOI: 10.35248/2167-0250.23.12.302
Copyright: © 2023 Brandeis J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.