Journal of Cancer Research and Immuno-Oncology
Open Access
ISSN: 2684-1266
ISSN: 2684-1266
Research Article - (2025)Volume 11, Issue 1
Vanishing Bile Duct Syndrome (VBDS) is a serious drug induced liver injury characterized by chronic cholestasis and loss of intrahepatic bile ducts. VBDS has been reported also following checkpoint inhibitor treatment. We compared CD3+, CD4+, CD8+, CD20+, CD56+, PD-1+ and PD-L1+ lymphocyte infiltrates in liver biopsies of patients that encountered VBDS (n=2) or hepatotoxicity (n=3) after pembrolizumab-(n=4) or nivolumab-(n=1) treatment with samples from normal liver (n=10), non-alcohol steatohepatitis (NASH, n=10), primary biliary cholangitis (PBC, n=10) or pembrolizumab-treated patients without adverse events (n=2). We also studied direct growth effects of pembrolizumab on primary human intrahepatic biliary epithelial cells (HIBEpiC) in vitro. Liver sections of all checkpoint inhibitor-treated patients exhibited significantly higher CD3+ infiltration than normal livers, and significantly higher PD-L1+, CD4+ and CD8+ infiltration, than other groups. PD-1+ infiltration was significantly increased in livers of patients with severe hepatic adverse event. CD57+ infiltration was similar in normal livers, NASH- and PBC-groups, but highly increased in the checkpoint inhibitor-treated patients. Immune cell infiltrates were similar between NASH and normal livers. PBC samples had significantly higher CD3+, CD4+, CD8+ and CD20+ infiltrates than normal livers. HIBEpiC express PD-L1 but pembrolizumab did not affect their viability in vitro. Our findings suggest that VBDS is not due to direct cytotoxicity of checkpoint inhibitors and that the immunological attack against livers induced by these drugs is different from other cholestatic liver conditions. Biological insight: Upregulation of PD-1 and PD-L1, as well as cytotoxic CD57+ cells may play a role in anti-PD-1 antibody induced hepatotoxicity.
Checkpoint inhibitors are monoclonal antibodies that target CTLA-4 (ipilimumab), PD-1 (nivolumab, pembrolizumab) or PD-L1 (atezolizumab, durvalumab), on T-cells or cancer cells. These drugs unlock the anti-tumor efficacy of oncolytic T-cells, enabling immunological destruction of tumors. Their clinical adoption has dramatically changed the course of various metastatic cancers. These drugs are also being increasingly studied and used also in earlier cancer stages [1].
Checkpoint inhibitors may also induce toxic side effects in healthy tissues, which are usually successfully managed with immunosuppressants. Some side effects may, however, occur rapidly and can be resistant to immunosuppressants [2].
Vanishing Bile Duct Syndrome (VBDS) is an example of a serious side effect associated with these drugs. To gain more understanding of VBDS pathophysiology, we studied immune cell infiltrates in liver biopsies of patients that had either a transient liver injury or fatal or non-fatal VBDS, or no adverse events in response to pembrolizumab or nivolumab and compared them with those from Non-Alcohol Steato Hepatitis (NASH), Primary Biliary Cholangitis (PBC) or normal livers [3-5].
Patient samples: We studied liver biopsies of patients, that experienced hepatotoxicity (n=5) or had no adverse effects (n=2) after treatment with pembrolizumab or nivolumab at the departments of oncology, university hospital of Oulu, university hospital of Kuopio, university hospital of Tampere, Finland, or at IRCCS humanitas research hospital, Rozzano, Milan, Italy. All patients were adults, none had liver metastases, hepatic viral infections or known contra-indications for checkpoint inhibitors. Information of the five patients that experienced a serious, treatment-associated hepatic side effect is given on Table 1. Samples of normal liver, NASH and PBC were obtained from the Borealis Biobank (Oulu, Finland). The Regional Ethics Committee of Northern Ostrobothnia Hospital District and the Medical Research Ethics Committee of Wellbeing Services County of North Savo approved this study.
| Patient # 1 | Patient # 2 | Patient # 3 | Patient # 4 | Patient # 5 | |
|---|---|---|---|---|---|
| Cancer-stage | Melanoma IV | RCC* III | Melanoma III | NSCLC** IV | Colon cancer IV |
| Checkpoint-inhibitor-# of treatments | Pembrolizumab 1 | Pembrolizumab 7 | Nivolumab 12 | Pembrolizumab 1 | Pembrolizumab 3 |
| Symptom onset -days after previous anti-PD-1 treatment |
1 | 67 | 48 | 20 | 39 |
| Immunosuppressants | Yes | Yes | Yes | No | Yes |
| Steroids | + | + | + | - | + |
| Myconophenolate | + | + | + | - | + |
| Days used prior to liver biopsy | 4 | 43 | 7 | - | 10 |
| Outcome | Fatal | Survived | Survived | Survived | Survived |
| Highest value | |||||
| Bilirubin | 655 | 401 | 269 | 752 | 123 |
| ASAT | 835 | 405 | 150 | 261 | - |
| ALAT | 1769 | 2137 | 364 | 818 | 916 |
| GGT | - | 1716 | 411 | 5148 | 1744 |
| ALP | - | 598 | 466 | 478 | 668 |
| INR | 3.5 | 1.1 | 1.1 | - | 478 |
| NH4 | 53 | 66 | - | - | 1 |
| Bile ducts vanished | Yes | No | Partial | Yes | No |
| Cholestasis | Yes | Yes | Yes | Yes | Yes |
Table 1: Characteristics of patients, that experienced checkpoint-inhibitor treatment associated hepatotoxicity.
Lymphocyte quantitation: Cut sections of liver biopsies were stained with antibodies against CD3 (NCL-L-CD3-565), CD4 (NCL-L-CD4-368), CD8 (NCL-L-CD8-4B11) all from Novo Castra Leica, CD20 (MO755, Clone L26, Dako), CD57 (Natural Killer Cell Marker, Thermoscientific), PD-1 (ab52587, Abcam) and PD-L1 (E1L3N, Cell Signaling) with standard immunohistochemistry. The stained slides were scanned with Leica Aperio Slide Scanner. The images were analyzed by QuPath positive pixel count, by calculating the percentage of immunopositive pixels of all pixels of the section, with the following settings: Downsample factor 2.0, Gaussian sigma 1 and DAB threshold 0.3 [6].
Cell viability assays: Primary Human Intrahepatic Biliary Epithelial Cells (HIBEpiC) were plated on 96-well plates (103 cells in 100 l) in manufacturer recommended normal growth medium (ScienCell Laboratories) per well and cultured at 37°C under standard conditions. The next day, vehicle, pembrolizumab, or doxorubicin (Selleck chemicals) were added to the cells. Cellular growth as a function of time was analyzed with MTS-assays, as previously described [7].
Western blotting: Human MDA-MB-231 breast cancer cells and murine J774 macrophages were obtained from ATCC, and cultured as usual. Peripheral blood mononuclear lymphocytes (donated by healthy volunteers and isolated with Ficoll) were extracted with RIPA buffer (Bio-Rad) and run on Novex™ 4-20% Tris-Glycine Mini Gels (Thermo Fisher), 25 g denaturated protein/lane. The separated proteins were transferred to nitrocellulose membranes, which were incubated with either anti-PD-1 (D4W2J) XP® Rabbit mAb #86163) or anti-PD-L1 (E1L3N®, XP® Rabbit mAb #13684) antibodies (diluted at 1:1000), followed with secondary antibody (1:30000, anti-rabbit IgG (H+L) (DyLight™ 800 4X PEG conjugate #5151, all from cell signaling technology). The membranes were scanned with LI-COR Odyssey using fluorescence at 800 nm. For loading control, the membranes were stripped and re-probed with betaactin loading control monoclonal antibody (BA3R, DyLight 680, Thermo Fisher Scientific, 1:1000) and scanned using fluorescence at 680 nm [8-10].
CD3+ tissue areas were significantly higher in the livers of anti- PD-1-antibody-treated patients, as compared with normal and NASH livers (Figure 1A). Livers from anti-PD-1-antibody-treated patients exhibited also statistically significantly higher CD4+, CD8+ and PD-L1+ tissue areas, as compared with the other groups (Figures 1B,C,G). CD20+ areas were similar in the anti- PD-1-antibody-treated and other groups (Figure 1D). CD57+ stained areas were higher among anti-PD-1 antibody treated patients than in the other groups. This difference reached statistical significance between the anti-PD-1-antibody-treated patients without adverse events and normal liver, NASH and PBC groups (Figure 1E). PD-1+ tissue area was statistically significantly higher among the patients that experienced a serious hepatic adverse effect from anti-PD-1 treatment, as compared with normal liver, NASH and PBC groups. Although the distribution of PD-1+ staining was higher in the group that experienced serious hepatic injury, the anti-PD-1 antibodytreated groups did not statistically differ in this regard. CD3+, CD4+, CD8+ and CD20+ areas were significantly higher in the PBC-group, than in normal livers (Figure 1). No differences were detected between NASH-and normal liver-groups (Figure 1A-G).
HIBEpiC cells express PD-L1, but not PD-1 (Figure 1 H-I). Pembrolizumab, tested at concentrations ranging from 10-fold below and above the therapeutic plasma concentrations, did not affect HIBEpiC cell viability in vitro. As expected, doxorubicin inhibited HIBEpiC viability (Figure 1).
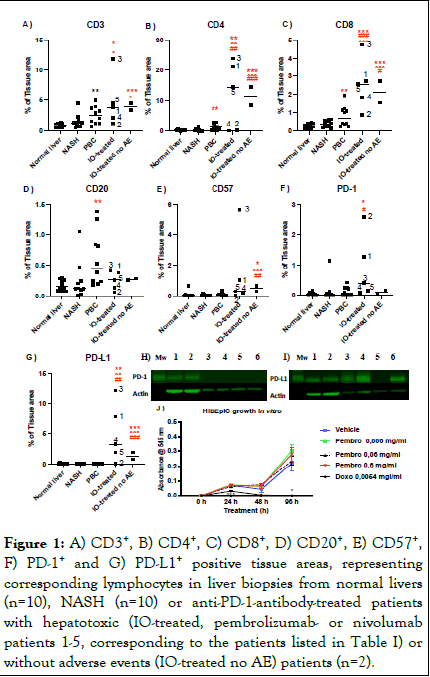
Figure 1: A) CD3+, B) CD4+, C) CD8+, D) CD20+, E) CD57+, F) PD-1+ and G) PD-L1+ positive tissue areas, representing corresponding lymphocytes in liver biopsies from normal livers (n=10), NASH (n=10) or anti-PD-1-antibody-treated patients with hepatotoxic (IO-treated, pembrolizumab- or nivolumab patients 1-5, corresponding to the patients listed in Table I) or without adverse events (IO-treated no AE) patients (n=2).
Individual values for each patient data points are shown, the transverse bars represent the mean value, * p<0.05, ** p<0.01, *** p<0.001 vs. normal livers, ˆ p<0.05, ˆˆp<0.01 ˆˆˆp<0.001 vs. NASH, # p<0.05, ## p<0.01, ### p<0.001 vs. PBC. H) PD-1 and I) PD-L1 protein expression in samples: 1-2) PBMC cells from volunteers, 3–4) human MDA-MB-231 breast cancer cells, 5) murine J774 macrophage cells and 6) HIBEpiC cells. B-actin of the same blots are shown to demonstrate protein loading. J) HIBEpiC viability as a function of time in the presence of indicated concentrations of pembrolizumab (pembro), doxorubicin (doxo) or vehicle (control). Data is expressed as mean + SEM, n=5, * p<0.05, *** p<0.001 vs. vehicle.
We compared liver immune cell infiltrates of pembrolizumab- or nivolumab-treated patients with or without treatment-associated hepatic adverse effects to those of normal livers, NASH and PBC. The most striking finding here was that PD-L1 expression was significantly higher in the liver sections of anti-PD-1 antibody-treated patients, as compared with other groups. Furthermore, PD-1+ was significantly increased among patients that experience treatment-induced serious hepatic adverse event, as compared with those that were treated without such adverse effect. Despite small sample size and the lack of pre-treatment control samples, these findings agree with previous reports describing increased PD-L1 expression in cancer tissues and peripheral blood T-lymphocytes after pembrolizumab-treatment. These findings further suggest that anti-PD-1 antibodies may increase the expression of their own target. As such, this finding would offer a plausible explanation as to why these drugs may induce favorable treatment responses in malignancies even when PD-L1 expression is initially absent. Further investigation is needed to define whether this phenomenon also contributes to the serious side effects associated with these drugs. Interestingly also, although none of the studied patients had liver metastases, there was a significant accumulation of CD3+, CD4+ and CD8+ lymphocytes into the livers with these treatments.
This work was funded by grants from the Thelma Mäkikyrö Foundation and the Jane and Aatos Erkko Foundation (K.S.S.). The authors wish to thank Ms. Erja Tomperi for the immunohistochemical stainings, Dr. Pia Österlund for finding a patient for this study and Dr. Kevin Harris for proof-reading the manuscript. Borealis Biobank is acknowledged for providing the samples.
Experimental design: KSS, KV
Laboratory experiments: MR, EP, SN
Clinical sample and patient information retrieval: KSS, AJ, LDT, HR, KK, AR, RK
Immunohistochemistry analyses: KV, MS
Writing: All authors.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Saarela M, Lleo A, Tommaso LD, Raunio H, Kankaanranta K, Vuopala K, et al. (2025) Increased PD-1 Expression in Livers Associated with Anti-PD-1 Antibody-Induced Hepatotoxicity. J Cancer Res Immunooncol. 11:239.
Received: 28-Aug-2023, Manuscript No. JCRIO-23-26314; Editor assigned: 01-Sep-2023, Pre QC No. JCRIO-23-26314 (PQ); Reviewed: 15-Sep-2023, QC No. JCRIO-23-26314; Revised: 14-Jan-2025, Manuscript No. JCRIO-23-26314 (R); Published: 21-Jan-2025 , DOI: 10.35248/2684-1266.25.11.239
Copyright: © 2025 Saarela M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.