Journal of Horticulture
Open Access
ISSN: 2376-0354
ISSN: 2376-0354
Research - (2019)Volume 6, Issue 3
This study was conducted to establish in vitro and ex vitro propagation systems and to secure the genetic resource for ex-situ conservation of Astilboides tabularis (Hemsl.) Engl. as a rare and endangered species. Plant materials were collected and prepared for various treatments for in vitro propagation by plant tissue culture and ex vitro propagation by root cuttings. For callus induction, leaf explants cultured on MS medium with BA 0.1 mg L-1 showed the highest rate at 24%, whereas the petioles supplemented with BA 0.5 mg L-1 exhibited the highest rate at 14%. Root explants treated with BA alone did not induce callus formation whereas a combination of BA and NAA showed 100% callus induction at concentrations of BA 0.1 + NAA 0.1 mg L1 and BA 1.0 + NAA 1.0 mgL-1. Shoots were produced at 50% from leaf explants treated with BA 0.1 mg L-1 concentration, whereas no shoots were observed even with the treatments from root segments. Rooting experiments supplemented with NAA 0.01 mgL-1 had the highest rate up to 86% with 15 small roots and 1.3 cm length. In order to increase the efficiency of in vitro potential regeneration of A. tabularis, additional studies would be needed for higher rooting rates from callus and induced shoot. Ex vitro propagation by root cuttings with large diameter and 7 cm length grew well above ground, whereas medium diameter and 7 cm length showed good rooting system in the first year. During the second year, small shoots grew well for medium diameter, whereas there were no significant differences among other treatments. Thus, the size and length of the cuttings were for the plants in the first year, which did not affect growth in the second year. Based on this work, A. tabularis root cutting for mass propagation would be possible by small size cutting system.
Astilboides tabularis; Callus; Conservation; Germination; Growth; In vitro and Ex vitro propagations; Root cuttings
As the world population increases, the natural environment is compromised by industrial development. Some native plants have become extinct or endangered due to disturbances in the natural ecosystem. Astilboides tabularis is an herbaceous perennial Rosales plant, belonging to the Saxifragaceae family. The species are distributed in the Northeast Asian region including China, Russia and Korea which are the Southern limit line in terms of habitat. A. tabularis has been designated as a rare and endangered species which grows in sloping areas where the roots are easily exposed and damaged. These plants grow up to 1∼2 m high. In the forest, the leaves are shield-shaped and the diameter growth is around 70∼80 cm and one of the plants on the ground with the largest leaves in Korea [1]. Several studies on A. tabularis have been conducted to investigate the environmental characteristics of its habitat in order to identify the impact of environmental conditions on seed germination [2,3]. However, more studies are needed on habitat protection because the plants are vulnerable to climate changes and habitat is an environment where morphological change can occur easily [4]. Therefore, in this study, we have developed a mass proliferation method used for in vitro plant tissue culture and ex vitro root cuttings in order to obtain genetic resources for ex-situ conservation of A. tabularis, which is distributed in native habitats with a high risk of individual loss.
In vitro propagation by plant tissue culture
Plant materials and culture conditions: The materials were collected at the end of September in 2014, near the top of Deokhang Mountain in Singi-myeon Samcheok-si Gangwon-province. Seeds show brown pericarps which means they are fully mature. After they were dried at room temperature for one week, they were kept at 4 ± 1°C until used. Culture conditions were maintained at a temperature of 24 ± 2°C with an illuminance of 40 μmol m-2s-1.
In vitro propagation with plant growth regulators: For in vitro seed germination, seeds were dipped in 70% ethanol (EtOH) for 30 seconds followed by sodium hypochlorite (4%) at concentrations of 0.2%, 0.4%, 0.6% and 0.8%. The seeds were immersed in the solution for 10 minutes and then washed five times with sterilized water. After germination, when the seedlings were >1 mm, they were transferred to ECO2 box green filter (125 mm × 65 mm × 80 mm) (Duchefa Biochemie Company. Seedlings were cultured in MS (Murashige and Skoog) basic medium containing 3% sucrose and 0.3% gelite. Leaf, petiole and root explants from the seedlings were used for callus induction. BA (Benzyladenine) was treated at four different concentrations (0, 0.1, 0.5, and 1.0 mgL-1). However, for the root culture, NAA (Naphthalene acetic acid) was additionally treated at 0.1, 0.5 and 1.0 mgL-1 concentrations. BA, NAA and IBA (Indole-3-butyric acid) are used for shoot introduction. Callus obtained from leaf explants were grown for 8 weeks and high concentration of BA (0.1, 0.5 mgL-1) induced friable callus for shoot formation. Mixed treatments included auxin with NAA and IBA at a cytokinin: auxin (10:1) ratio. The roots were further treated because BA was effective at 1.0 mgL-1. The experiment was repeated 5 times with 5 replicates per Petri dish with subcultures conducted at periods of 2 weeks. The shoots were divided into 2 or 3 explants and placed on 0.01, 0.05, 0.1, and 0.5 mgL-1 concentrations of NAA. To investigate the growth of the shoots, 5 pieces were planted in each magenta box (72 mm × 72 mm × 100 mm) and the treatment was repeated 3 times.
Ex vitro propagation by root cuttings
Plant materials: Astilboides tabularis (Hemsl.) Engl. cutting materials were collected at the end of September 2014, near the top of the Deokhang Mountain in Singi-myeon Samcheok city, Gangwon-Province. There are one or two stems out from the main root and about 30 root explants were obtained from this cutting. The collected cuttings were washed with tap water and wrapped with paper and used after 3 days. The experiment was conducted in the greenhouse (with 50% shading film) at the Useful Plant Propagation Center of Korea National Arboretum in Yangpyeong-gun, yongmun-myeon, Gyeonggi-Province. Automatic irrigation was applied for 15 minutes once a day in the mornings. Average temperature and humidity inside the greenhouse were measured using Acuba cs-201 (Chosun Machine Company).
Root cutting methods: The experiment was conducted between June to August 2014, and the experiment was not repeated due to the sample being a rare endangered species, the availability of raw materials is limited. In order to investigate the effects of diameter and root length, the cutting materials were divided into large, medium and small lengths of 7, 5 and 3 cm, respectively. The average diameter for large was 39.53 mm, medium 31.79 mm and small 24.13 mm. Fifteen cutting materials were prepared per specimen and planted at 2 cm intervals. “Kanuma soil (OOBARi Company)” was used for the root cuttings which were placed at regular intervals and covered up to 1 cm.
Statistical analysis
The data collected in this experiment were calculated as means ± standard deviation error, and the differences between groups were investigated. The significance was tested by Duncans Multiple Range test (p=0.05). All statistical analysis was performed using SPSS Version 21 (IBM Corp., Korea).
In vitro propagation by plant tissue culture
For in vitro germination with different concentrations of ethanol, seeds of A. tabularis sterilized with 70% ethanol had the highest germination rate at 40%. Serial disinfection with NaOCl 0.8% (stock solution 4%) had the highest germination rate of 40.7%. Pre-treatment with ethanol is recommended to avoid high contamination risks in in vitro germination. But high concentrations of ethanol increase the risk of seed dehydration since germination was not achieved when 100% ethanol was used for pre-treatment [5]. Also, a second sterilization was required to dilute concentrations since high concentrations of NaOCl damaged the thin seed coat [6]. The previous study with germination experiments based on temperature conditions and PGRs showed a high germination rate of 97% at 30°C under a light condition with treatments GA3 200 and 500 mgL-1 [3]. These results suggested that germination rate can be promoted if additional studies are carried out using plant growth regulators to promote germination in in vitro condition. Callus and shoot explants were induced from leaf and petiole with BA alone, however, the root explants induced callus with the addition of NAA. Leaf explants cultured on MS medium with BA 0.1 mgL-1 showed the highest rate at 24% of callus, whereas the petiole treated with BA 0.5 mgL-1 exhibited the highest rate at 14% (significantly different p<0.01) (Figure 1). This can be seen particularly in many herbaceous plants, in the case of Begonia usually in leaves [7]. Even though only cotyledon segments were used in In vitro experiments of watermelon varieties, the more effective variety was about shoot induction when treated with BA than the combination treatment with BA and IAA, where the results are similar to A. tabularis [8]. In various in vitro tissue, culture studies shoot induction rates were higher with a combination of auxin and cytokinin where A. tabularis showed different patterns with PGR effects considerably differing depending on the variety and tissue utilized [9,10]. In particular, some petioles produced shoots directly without callus induction (Figure 2). The root explants showed the highest rate of 100% for callus induction in the following concentrations NAA 1.0 mgL-1, BA 0.1 + NAA 0.1 mgL-1, BA 1.0 + NAA 1.0 mgL-1. NAA 1.0 mgL-1 and combined concentrations of BA 0.1 mg L-1 with NAA 0.1, 0.5, and 1.0 mg L-1 formed callus more actively than other conditions covering the entire root (Figure 2). Eight weeks after culture, callus obtained from leaves were placed on media with PGR; shoot formation began after 2 months, where the highest rate of 50% was induced with BA 0.1 mg·L-1 treatment. The second highest rate of 46% was with BA 0.5 mgL-1 treatment (significantly different p<0.02) (Figure 3). In root cultures, the callus was induced at a higher rate whereas the shoot induction rate was low. In the case of Cacalia firma In vitro experiments, which have a similar habitat and morphological structure, the shoot was induced in both leaves and petioles but not in the root [11]. These results suggest that the use of leaf and petiole as a starting material to maximize the efficiency of propagation can be achieved by taking advantage of the characteristics of A. tabularis; large leaf lengths and long petioles. The rooting test with NAA 0.01 mgL-1, had a rooting rate of 86% with 15 small roots, 1.3 cm in length (Table 1). Callus was induced in NAA 0.5 mgL-1 and root growth was slow. Roots were also observed in control and the root elongation was best, however, it is considered to be more effective with lower NAA concentrations. The rooting rate was higher, 50% for shoot induction and the callus induction effect requires further studies especially on callus and shoot induction.
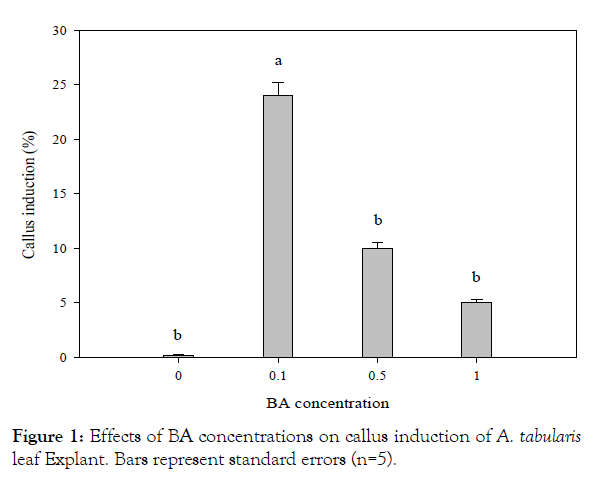
Figure 1: Effects of BA concentrations on callus induction of A. tabularis leaf Explant. Bars represent standard errors (n=5).
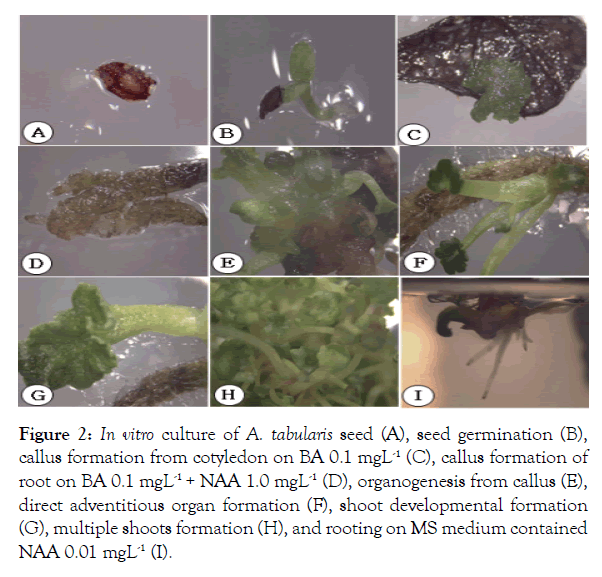
Figure 2: In vitro culture of A. tabularis seed (A), seed germination (B), callus formation from cotyledon on BA 0.1 mgL-1 (C), callus formation of root on BA 0.1 mgL-1 + NAA 1.0 mgL-1 (D), organogenesis from callus (E), direct adventitious organ formation (F), shoot developmental formation (G), multiple shoots formation (H), and rooting on MS medium contained NAA 0.01 mgL-1 (I).
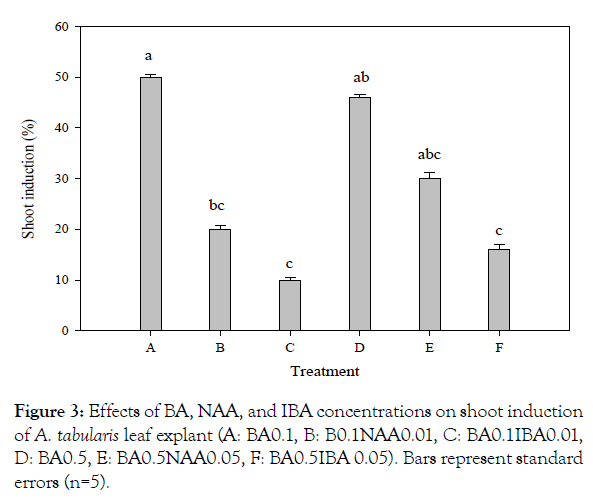
Figure 3: Effects of BA, NAA, and IBA concentrations on shoot induction of A. tabularis leaf explant (A: BA0.1, B: B0.1NAA0.01, C: BA0.1IBA0.01, D: BA0.5, E: BA0.5NAA0.05, F: BA0.5IBA 0.05). Bars represent standard errors (n=5).
| NAA (mg.L-1) | Rooting (%) | No. of roots | Root length (cm) |
|---|---|---|---|
| 0 | 6.6 ± 0.33bz | 2.6 ± 2.60c | 0.4 ± 0.40b |
| 0.01 | 86.6 ± 0.33a | 15.0 ± 2.91a | 1.3 ± 0.07a |
| 0.05 | 66.6 ± 0.33a | 10.6 ± 1.50ab | 0.9 ± 0.07ab |
| 0.1 | 80.0 ± 0.58a | 10.4 ± 1.21ab | 0.6 ± 0.06b |
| 0.5 | 66.6 ± 0.33a | 5.4 ± 0.75bc | 0.4 ± 0.04b |
zMean ± SE (Superscripted letters are significant at p<0.05)
Table 1: Effect of NAA concentrations on A. tabularis rooting.
Ex vitro propagation by root cuttings
The maximum and minimum greenhouse temperature in June was 29°C and 18°C, with the maximum and minimum humidity was 87% and 52%. Whereas in July the maximum and minimum greenhouse temperature were 32°C and 20°C, with the maximum and minimum humidity at 87% and 52%. In regard to initial root cuttings, good levels of shoots were induced in both large and medium-size shoot diameters. Particularly, when it comes to root cuttings of A. tabularis, it was not the diameter but length that affects shoot induction because the results showed shoot induction rate of 100% in overall 7 cm long cuttings. This was consistent with the study by Shin where growth changes of A. tabularis were dependent on rhizome size [12]. In particular, the growth characteristics of the ground parts were better as the cutting length increased, which are similar to previous studies [13-15]. In 1 year survey of aboveground growth characteristics, the best was observed in large diameter and 7 cm length with the following details: number of leaves 4, leaf length 12.14 cm, leaf width 14.28 cm, aboveground fresh weight 11.88 g (Table 2 and Figure 4). The belowground growth characteristics were best in medium diameter and 7 cm length with the following details: number of roots 47.6, belowground fresh weight 2.41 g (Table 2). On the other hand, the lowest diameter of 3 cm had the lowest values except for leaf number and root length across all lengths (Table 2). Generally, new root developments are induced from original roots and these induce latent lateral roots from the original roots [16]. In root cutting studies of Detarium microcarpum, large sizes grew vigorously when cutting sizes were differentiated [17,18]. In the second year, 7 cm length cuttings of all diameter sizes which were active in the first year were damaged or had poor growth. Based on the fact that the cutting explants weighed more with time, it is because cuttings were transferred and planted into small pots, hence they were restricted from growing.
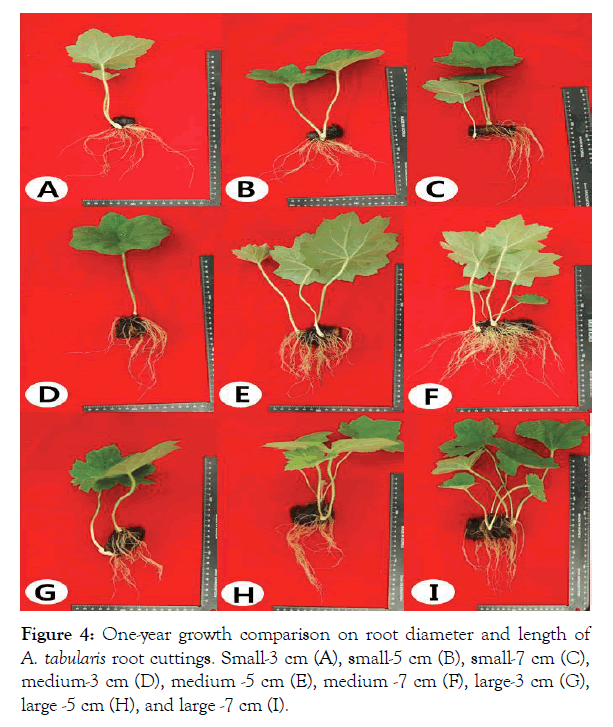
Figure 4: One-year growth comparison on root diameter and length of A. tabularis root cuttings. Small-3 cm (A), small-5 cm (B), small-7 cm (C), medium-3 cm (D), medium -5 cm (E), medium -7 cm (F), large-3 cm (G), large -5 cm (H), and large -7 cm (I).
| Size | Length (cm) |
No. of leaves |
Leaf length (cm) |
Leaf width (cm) |
Petiole length (cm) |
No. of roots |
Root length (cm) | Fresh weight(g) | |
|---|---|---|---|---|---|---|---|---|---|
| Above Ground |
Below Ground | ||||||||
| Sz | 3 | 2.80 ± 0.38abw | 7.14 ± 0.78c | 8.50 ± 0.71d | 8.94 ± 0.76d | 18.20 ± 2.60d | 11.20 ± 0.33bc | 3.33 ± 0.30e | 0.72 ± 0.10d |
| 5 | 2.40 ± 0.25b | 8.60 ± 0.42bc | 10.26 ± 0.59cd | 11.50 ± 0.84bc | 22.00 ± 4.16d | 12.68 ± 0.69b | 4.44 ± 0.74de | 0.90 ± 0.15cd | |
| 7 | 3.00 ± 0.71ab | 9.12 ± 0.86bc | 10.82 ± 1.19bcd | 10.48 ± 0.88cd | 33.60 ± 4.76bcd | 11.74 ± 0.49b | 6.54 ± 0.73cd | 1.55 ± 0.13bc | |
| My | 3 | 2.00 ± 0.32b | 10.22 ± 0.60ab | 12.68 ± 0.84abc | 13.24 ± 0.49ab | 28.40 ± 3.33bc | 10.88 ± 0.52bc | 5.88 ± 0.68cde | 1.03 ± 0.09cd |
| 5 | 2.40 ± 0.25b | 10.58 ± 0.61ab | 13.34 ± 1.05ab | 13.40 ± 0.84ab | 31.60 ± 3.67b | 12.58 ± 0.39b | 7.91 ± 0.89bc | 1.53 ± 0.34bc | |
| 7 | 4.00 ± 0.45a | 10.64 ± 0.64ab | 12.40 ± 0.94abc | 14.20 ± 0.93a | 47.60 ± 8.45a | 12.68 ± 0.70b | 9.98 ± 1.30ab | 2.41 ± 0.34a | |
| Lx | 3 | 2.00 ± 0.00b | 11.88 ± 1.50a | 14.24 ± 1.55a | 13.26 ± 1.09ab | 20.20 ± 1.39cd | 9.70 ± 0.70c | 5.52 ± 0.88cde | 1.14 ± 0.15bcd |
| 5 | 4.00 ± 0.63a | 10.80 ± 0.6ab | 13.54 ± 0.55ab | 13.42 ± 0.62ab | 40.80 ± 6.12b | 11.84 ± 0.59b | 9.40 ± 1.07ab | 1.78 ± 0.19ab | |
| 7 | 4.00 ± 0.55a | 12.14 ± 0.64a | 14.28 ± 0.55a | 14.14 ± 0.68a | 38.20 ± 7.66b | 15.84 ± 0.42a | 11.89 ± 0.62a | 2.22 ± 0.31a | |
zSmall, yMedium, xLarge, wMean ± SE (Superscripted letters are significant at p<0.05)
Table 2: One-year growth data of A. tabularis root cuttings.
When comparing the growth in the first and second year, the overall growth of the second year had improved.
Although there were significant differences (p<0.05) between the two conditions in the first year of growth, and the size and length of the cuttings were expected to affect A. tabularis, however, there was no significant difference in the second year of growth. According to Shin et al. large size cuttings of A. tabularis grew vigorously in the first year whereas small size cuttings showed large differences in growth change over time. Finally, it can be said that the root cuttings of A. tabularis were not affected by the size of the cuttings because petiole length which indicated the height of herbaceous plants was highest in small diameter (data not shown). In conclusion, it is considered that the size and length of the cuttings do not affect A. tabularis. In addition, analysis of the growth conditions in the first and second year of A. tabularis, root cuttings showed that small size and short lengths improve the shooting efficiency, especially within a limited habitat.
Citation: Shin U, Chandra R, Kang H (2019) In vitro and Ex vitro Propagations of Astilboides tabularis (Hemsl.) Engl. as a Rare and Endangered Species. J Horttic 6:261
Received: 21-Sep-2019 Accepted: 12-Oct-2019 Published: 21-Oct-2019
Copyright: © Shin U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.