Journal of Proteomics & Bioinformatics
Open Access
ISSN: 0974-276X
ISSN: 0974-276X
Research Article - (2008) Volume 1, Issue 2
The present study supports previous finding that vitamin A can induce and accelerate lens regeneration in pigmented epithelial cells (PECs) of dorsal iris not only in amphibians but also in young and adult swiss albino mice, guinea pig, rabbit and pigs. In lens regeneration, several workers have shown that vitamin A possesses the mitogenic activity which causes functional impairment of retinoid receptors and thereby inhibits the lens regeneration. The purpose of present study is to know how retinoids and their derivatives interact with Retinoid X receptor (Rxr alpha) and thus helping in lens regeneration. The docking studies of human and mice Rxr alpha were performed against vitamin A and 9-cis retinoic acid (vitamin A1) using autodock and the results were analyzed using Discovery Studio from Accelrys. The results show that there is a significant similarity in interaction energy of Rxr alpha of mice and human. The highest rank docked energy of Rxr alpha mice with vitamin A was -11.65 kcal, which was much closed to -11.83 kcal of 9-cis retinoic acid. Similarly, in human Rxr alpha receptor, the highest docked energy showed the significant similarity with vitamin A (-12.19 kcal) and 9- cis retinoic acid (-12.14 kcal). This study suggests that vitamin A shows effect on proliferation and differentiation similar to the 9-cis retinoic acid and also proves that vitamin A acts on Retinoid X alpha receptors and enhance lens regeneration in mammals.
Keywords: Docking, Vitamin A, 9-cis retinoic acid, Rxr alpha receptor, Lens regeneration.
Lens regeneration provides a clear example of trans-differentiation of one differentiated cellular type having a distinctive pattern of metabolic activities to another cellular type, which is morphologically and biochemically distinct from the original. An abundant literature exists on lens regeneration in amphibians (Reyer, 1971; Reyer, 1990; Stone, 1959; Yamada, 1967; Jangir et al., 1995). Lens regeneration from non ocular tissue (dorsal iris) has been well documented in amphibians (Reyer, 1954; Reyer, 1977; Eguchi and Itoh, 1982; Eguchi, 1988). However, the capability of iris and retinal pigmented epithelial cells (PECs) to trans-differentiate into lens In vitro is not restricted to urodeles only but is widely conserved in almost all vertebrates, including mammals. Although good lens regeneration from PECs in vivo has been seen in a few species of fishes and urodele amphibians. In cell culture, PECs of almost all vertebrates can switch differentiation to acquire the characteristics of lens (Eguchi, 1997; Okada, 2000). The role of retinoic acid in embryonic and post embryonic development has also been described. (Maden M, 2000). It has been found that expression and role of retinoic acid receptor alpha was very important in lens regeneration (Tsonis et al., 2002). Retinoid (vitamin A) are crucial for most forms of life. They have important roles in the developing nervous system and notochord and many other embryonic structures, as well as in maintenance of epithelial surfaces, immune competence, and reproduction. The ability of all-trans retinoic acid to regulate expression of several hundred genes through binding to nuclear transcription factors is believed to mediate most of these functions. (Rune, 2006) Experimentally, regenerative ability can be activated by using certain chemical likes vitamin A and 3-nitrobenzothiazolo (3, 2-a) quinolinium chloride (NBQ).Scientists has inferred that vitamin A and its derivatives were found to accelerate lens regeneration not only in amphibian frogs but also in mammals like swiss albino mice, rabbit, guinea pigs and pigs (Shekhawat et al., 2001).It has been reported that vitamin A (Retinol) is the immediate precursor to two important active metabolites: Retinal (that is important for vision) and Retinoic acid (which serves as an intracellular messenger that affects transcription of number of genes). Exogenous application of retinoic acid can regulate in a specific manner in the expression of number of homeobox genes implicated in lens regeneration (Maden, 1993). Retinoic acid is lipophilic signaling molecule that is synthesized from ubiquitous non-signaling precursor retinol and functions by activating nuclear receptor that are ligand dependent transcription factors present in responsive cells. The level of retinoic acid in a cell is regulated by cytoplasmic retinoic acid binding protein that may function in intracellular buffers. It has been reported that at molecular level the retinoic acid receptors are members of super family of intracellular hormone receptor which functions as regulator of gene transcription. These receptors comprise two distinct families, the retinoic acid receptor (RAR) family and the Retinoid X receptor (RXR) family; both families have subfamilies which have alpha, beta and gamma receptor proteins (Ralff and Ribeiro et al., 1995). The Retinoid X alpha receptors are nuclear receptors that mediate the biological effects of retinoid by their involvement in retinoic acid-mediated gene activation.
These receptors exert their action by binding, as homodimers or heterodimers, to specific sequences in the promoters of target genes and regulating their transcription (Khan, 2003). In our previous finding (Shekawat et al., 2001), it has been reported that vitamin A causes induction and acceleration of lens regeneration in young and adult mice. Vitamin A metabolite 9-cis retinoic acid present endogenously and works as an antagonist (Carlberg et al., 1993). These known properties of Rxr alpha, 9-cis retinoic acid and vitamin A motivated the present work to explore the influence of vitamin A on lens regeneration in young and adult mice. Recent methodologies like In silico docking and simulation studies were considered beneficial for this research as it provides the result with more accuracy in relatively very less time.
For the present experimental study swiss albino mice were employed. The experiments were carried out on newly born young mice (10 day old) and sexually matured adult mice (60 day). Lensectomy was carried out on 80 animals under local anesthesia (2% xylocaine). A longitudinal slit was made in the cornea of the right eye under a stereoscopic binocular microscope. The complete intact lens along with lens capsule was extracted through the incision. Following the operation, 30 IU/ml solution (young mice) and 50 IU/ml solution (adult mice) of vitamin A was injected intra peritoneal (I.P) on alternate days. In the case of 40 operated the animals of the control group were given Sham injections. The eyes were sectioned and stained with haemotoxylin and counter stained with eosin. Routine steps in the above process were followed according to Humason (1972). The experiments were terminated on day 40 after the operation.
For the present computational study, the protein structures of Rxr alpha receptor of human (PDB id, 1fby) and mice (PDB id, 1mv9) were obtained from Protein Data Bank. Four docking studies were performed. The Rxr alpha receptor of mice (1mv9) and human (1FBY) were docked with vitamin A and 9-cis retinoic acid. Discovery Studio Package from Accelrys (2.0) and AutoDock 4 from Scripps Research Institute were used for computational analysis and docking studies.
Initially, Discovery Studio Package was used to generate H-bonds on ligand and receptor. The CVFF91 forcefield were applied. In order to create dlg files for docking studies in AutoDock, solvation and grid parameters were applied.
In vivo studies elucidate all the experimental process. First of all swiss albino mice were treated with vitamin A. In young albino mice 90% and in adult albino mice 80% regeneration was observed. The docking of Retinoid X receptor alpha of human and mice with vitamin A and 9-cis retinoic acid were performed in silico. Looking into the similarities between docking results of Rxr alpha receptor of Human (1FBY) and Mice (1MV9), it can be inferred that vitamin A may accelerate lens regeneration in human also. However the percentage of lens regeneration in young and adult operated mice of control group was found low. It was 20% in young mice and only 2% in adult mice.
Histological study revealed that during lens regeneration after lensectomy the two layers of pigmented epithelium of the dorsal iris began to thicken and a cleft develops between two lamina of the dorsal iris (Figure 5 and Figure 6) and the nuclei of iris cells changed their shape. Then the pupillary margin of the iris becomes knob-like. The formation of this knob-like structure continued until the free margin became a swollen loop-like structure. Scattered mitotic figures were also observed. All these changes continue up to day 7 after operation in vitamin A treated animals. Then the cells started to dedifferentiate: they threw out their melanosomes. These melanosomes are ingested by macrophages that entered from the wounded site. Dorsal iris cells continued to divide, forming a vesicle-like structure in the region of the removed lens (Figure 7).
Now the vesicle differentiated into a new lens. Once the new lens formed, the cells of the dorsal iris ceased mitosis.
The newly formed lens was surrounded by a lens epithelium whose cells were cubiodal and slightly taller than before. In addition lens fiber formation was initiated in the inner surface of the vesicular lens. Cells began to elongate and entered the lumen of the vesicle. Gradually the lumen was filled by primary lens fiber nuclei (Figure 8) before the secondary lens fibers began to form.
Later on the secondary lens fibers began to differentiate and grow around the central nucleus and the regenerated lens became a better-defined structure (Figure 9 and Figure 10).
In the next stage the lens detached from the dorsal iris and returned to its normal status.
In silico studies explain all computational process. After successful docking, the resulted dlg files were analyzed which contain top ten ranked conformers with their respective docked energies. The docked structure with all top ranked conformers was visualized in Discovery Studio. The docking energy for IMV9 with vitamin A ranges between -11.65 kcal and -6.88 kcal and for 9-cis retinoic acid it ranges between -11.83 cal and -6.55 kcal. Similarly for 1FBY the docking energy for vitamin A was between -12.19 kcal and -7.30 and for 9-cis retinoic acid it was between -12.14 kcal and -4.10 kcal. The result showed that the docking energies of highest ranked conformer for Rxr alpha receptor of human with vitamin A and 9-cis retinoic acid were very close and similar was in case of mice. The structural and docked energy similarity of both receptors gives clear evidence why exogenous vitamin A proved beneficial for lens regeneration. The results of docking studies are mentioned in Table 2,3,4,5 and Figure 1,2,3,4 respectively.
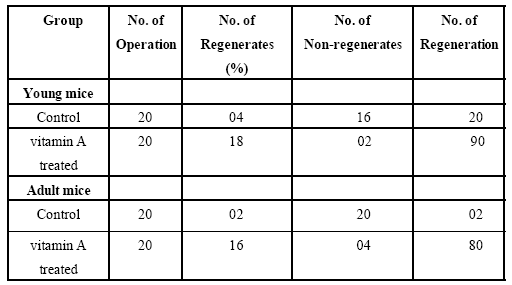
Table 1: Percentage of lens regeneration in young and adult mice of control and vitamin A treated groups.
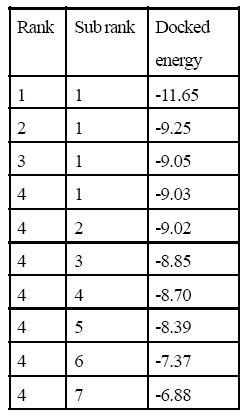
Table 2: 1MV9 Interaction with vitamin A.
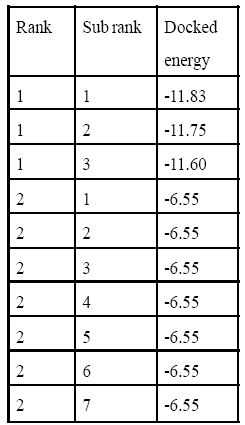
Table 3: 1MV9 Interaction with 9-cis retinoic acid.
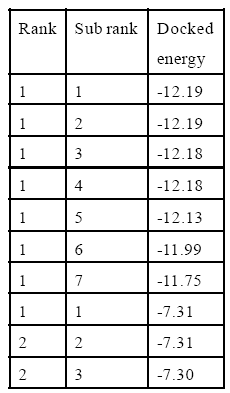
Table 4: 9-cis Retinoic acid Interaction with 1fby.
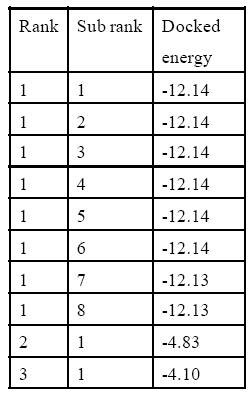
Table 5: vitamin A Interaction with1fby.
The main finding of this study is that mice have the capacity to regenerate an eye lens at early stages of development and this ability can be significantly enhanced by vitamin A treatment. The Retinoic acid receptors are super family of inter cellular hormone receptor. These receptors comprise two distinct families, the RARs and the RXRs. Both families have three subfamilies Rxr alpha, Rxr beta and Rxr gamma (Ralff and Ribeiro et al., 1995). Retinoid X alpha receptor is a nuclear receptor that mediates the biological effects of retinoid by involving in retinoic acid-mediated gene activation. These receptors exert their action by binding, as homodimers or heterodimers, to specific sequences in the promoters of target genes and regulating their transcription (Khan, 2003). The 9-cis retinoic acid also reported to be found endogenously and work as an antagonist (Carlberg et al., 1993). Retinoid X alpha receptor was found in outer neuroblastic layer (ONBL); retinal pigment epithelium (RPE); periocular mesenchyme (PM), lens, cornea and iris of prenatal mouse (Mikiro et al., 2001). This strongly proved the presence of Rxr alpha receptor in dorsal iris epithelium of mouse. Retinoic acid and Retinoid X receptor can induce the accumulation of B-crystalline in N/N1003A lens cells (Rashmi, 1998).
The in silico study of 9-cis retinoic acid and vitamin A were performed using Discovery Studio and Autodock tool. From the results it can be inferred that vitamin A shows effect on dedifferentiation, proliferation and differentiation similar to 9-cis retinoic acid. The docking results show that there is a significant similarity between the interaction energies of both the compound against Rxr alpha receptor. The 9-cis retinoic acid is a natural ligand of Rxr alpha receptor and has highest affinity towards this receptor. It has been reported that vitamin A doses enhance 9-cis retinoic acid concentration in the plasma of preruminant calves (Brian et al., 2000) can prove this theory that vitamin A can convert to its derivative 9-cis retinoic acid
The obtained results support the hypothesis that lens regeneration might be possible in human as the 1FBY, Rxr alpha human, was found to be interacted exactly in similar way as 1MV9, Rxr alpha mice. The structural and functional similarities between 9- cis retinoic acid and vitamin A make these molecules to be used as alternatively (Gaemers et al., 1998) and the 9-cis retinoic acid effecting proliferation, differentiation and expression on Rxr alpha using HL-60 cell (Kizaki et al., 1993). Another mechanism of vitamin A action reported that it may modulate the expression of RC3 mRNA by affecting RAR-alpha, RAR-beta and RXR-beta receptors (Mao, 1993). It has been found that expression and role of retinoic acid receptor alpha was very important in lens regeneration (Tsonis et al.,2002) and the role of retinoic acid in embryonic and post embryonic development was described (Maden M, 2000). It has been reported that exogenous application of retinoic acid can regulate in a specific manner the expression of a number of homeobox genes implicated in pattern generation (Mohanty- Hejmadi et al.,1992; Maden, 1993).
Another pathway of RA action might be through the influence on fibroblast growth factor (FGF). Recently it has been shown that FGF can initiate cell cycle events and cell division in the dorsal iris cells which are the basis for lens regeneration (McDevit et al., 1997). Recently it has been shown that following lensectomy, FGFR-1 is specifically present in the differentiating pigment epithelial cells of the dorsal iris and absent from the ventral iris. This may be the reason for lens regeneration from the dorsal iris (Tsonis et al., 2000) and has identified similar signatures of gene expression in dorsal and ventral with several cases of even higher levels in the ventral iris (Makarev et al., 2008). In the present study, vitamin A enhanced lens regenerative ability in young and in adult mice (90% in treated young mice in comparison to 20% in controls of the same age; no lens regeneration occurred in the adults of control group, while it was in 80% cases of adult treated animals). There is good evidence that vitamin A promotes and increases dedifferentiation in regenerating systems (Maden, 1993; Niazi, 1996). The Retinoids are thus group of chemicals that can be employed for investigations of the molecular mechanisms responsible for homeotic transformation. Thus from the present in vivo and in silico findings it can be concluded that vitamin A accelerating lens regeneration in mice.
We are thankful to BTIS Centre funded by Department of Biotechnology, Government of India for providing infrastructure facilities at BISR. Also, thankful to Developmental Biology Lab, Dunger College, Bikaner for the experimental support.