
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2025)Volume 14, Issue 2
Background: Structural and functional disorder dysfunction often does not have obvious symptoms in Type 1 Diabetes (T1D). However, exercise can improve cardiac performance in children with T1D, and its efficacy is directly related to index glycemic. The purpose of this study was to examine the 1) effect of 12 weeks of Interval and Resistance Training (IRT), and 2) 1 month of detraining on the cardiac structure and myocardial performance of adolescent males with T1D.
Methods: 24 adolescent males with T1D (FBS: 274.67 ± 52.99, age: 15.20 ± 1.78) and 12 healthy (FBS: 90.75 ± 5.47, age: 15.08 ± 1.67), were divided into three groups: Healthy Control (HC), Diabetes Control (DC), and Exercise Diabetes (DE). DE group performed 12 weeks of three times per week IRT. Blood glucose indices, echocardiography parameters, and peak oxygen consumption (VO2peak) were measured in base conditions, after the exercise intervention, and in the 1 month detraining period. The statistical method used for data analysis was repeated measures ANOVA.
Results: In the DE group, the Hemoglobin A1c (HbA1c) and Fasting Blood Sugar (FBS) significantly decreased, and VO2peak increased dramatically after intervention (P<0.05). Variables of Ejection Fraction (EF), Fractional Shortening (FS), early diastolic inflow velocity (E) tricuspid, and velocity during active atrial contraction (A) tricuspid significantly increased, but left ventricular internal diameter, Deceleration Time (DT) tricuspid, and DT mitral significantly decreased (P<0.05). HbA1c levels, left ventricular diameter, DT tricuspid, E tricuspid, A tricuspid, EF, and VO2peak remained significantly improved compared to baseline after detraining (P<0.05).
Conclusions: In adolescent's males with T1D, who undertook intervention, training associated with improved heart cardiac structure and performance, may have induced adaptations in cardiac anatomy and performance, and appears to be partially reversible in this age group.
Type 1 diabetes; Echocardiography; Adolescents; VO2peak; Interval and resistance training
Type 1 Diabetes condition (T1D) is a chronic metabolic and endocrine disease commonly diagnosed in youth [1], it can manifest in individuals of any age and most individuals living with T1D are adults. The incidence and prevalence of T1D have increased, and subclinical complications related to T1D are common [2]. Studies in adults with T1D have reported structural and performance disorders of the myocardium [3]. In addition, significant changes in the dimensions of the ventricular cavity and myocardial heart relaxation of young people with T1D have been observed [4], along with diastolic abnormalities [5]. Diastolic dysfunction is a condition that affects the heart's ability to relax during early diastole, causing changes to the way ventricular filling patterns occur. This can lead to an increase in left ventricular filling pressure during exercise and a decrease in resting and exercising end-diastolic volume [6]. The progression of asymptomatic preclinical damage in people with T1D may lead to the appearance of symptoms in diabetes-related cardiomyopathy, as well as heart failure with metabolic damage and abnormalities in the dimensions and performance of the myocardial [7]. So that the dysfunction of the heart of people with diabetes may worsen with age [8]. Previous studies in diabetic adults showed that physiological growth, myocyte size, pathological remodeling, LV wall compliance, contraction relaxation velocity, EF, and cardiac output, are improved with exercise in adults with T1D [9-11]. It's encouraging that exercise can have such a positive impact on the health outcomes of those living with T1D. Careful monitoring and treatment of this condition are important to maintain overall heart function. The detectable functional abnormality in cardiomyopathy initially begins with diastolic dysfunction, which may then progress to systolic dysfunction [12]. Echocardiography has been used as a noninvasive means to detect early heart functional abnormality in T1D [13], with Doppler echocardiography being the most reliable method to evaluate the filling of the ventricles and cardiac structural changes [14]. Detection of early changes in heart structure and function in youth with T1D may lead to noninvasive interventions to prevent and control future complications.
People with type 1 diabetes have lower maximum oxygen consumption (VO2peak) compared to healthy people, which is a reliable and repeatable index for evaluating cardiorespiratory system performance and large muscle groups [15]. Regular exercise may offer a non-pharmacological solution to limit potential changes in cardiac function and performance in younger individuals with T1D due to its myriad metabolic and physiological benefits, including enhanced hemodynamic regulation in circulation [16].
However, few people with T1D use the benefits of exercise due to the fear of hypoglycemia in aerobic exercise [17] and hyperglycemia in strength exercise [18] and insufficient knowledge about exercise management in a safe range.
However, it appears that a suitable and effective exercise protocol for adolescents with T1D that includes safe type, intensity, and duration, as well as consideration of glycemic control and the benefits of cardiac aerobic, structural, and functional capacity, has yet to be established. The purpose of the present study was to compare the cardiac structure and MPI of adolescent males with T1D before and after 12 weeks of Interval and Resistance Training (IRT) using Doppler echocardiography. Also, the durability of cardiac adaptations to exercise training was evaluated after one month of detraining.
This study included 24 adolescent males with T1D and 12 healthy male subjects aged between 12 and 18 years, who were divided into three groups age-homogeneous: Healthy Control (HC), Diabetes Control (DC), and Exercise Diabetes (DE) (Figure 1). The DC and DE groups had an average of 3 times a day, 55.24 (28.08 rapid-acting, 27.16 basal), and 53.49 (27.16 fastaction, 26.33 short-action) units of insulin injections per day, under the supervision of an endocrinologist. All subjects were students of Hamadan Province, who were in growth stages 3–5 of Tanner’s scale (based on the size of the penis, testes, and scrotum) and were considered inactive based on the number of daily steps (<5000 step/day) [19,20]. The boys were encouraged to follow a balanced and healthy diet under the supervision of a specialist and to record their daily energy intake (2091 to 2758 kcal/day). The control groups did not have a special training program except daily physical activity, monitored with a pedometer. The inclusion criteria for the study required participants to have a history of diabetes for at least 6 years, a body mass index of less than 25 kg/m2, fasting blood sugar levels of over 130 mg/dl, and hemoglobin A1C levels of over 7.5%. However, those with a history of cardiopulmonary disease, neuromuscular or metabolic abnormalities, those taking drugs that affect heart function, or those who were noncompliant with exercise and nutrition programs were excluded from the study. These findings are summarized in Table 1. The study was approved by the Research Ethics Committee of Bu-Ali Sina University-Hamedan for humanities studies (Approval ID. IR.BASU.REC.1400.040, IRCT202111031052926N1) and, therefore, has been performed under the ethical standards laid down in the 1964 declaration of Helsinki. Written Informed consent was obtained from all subjects and their parents prior to participation.
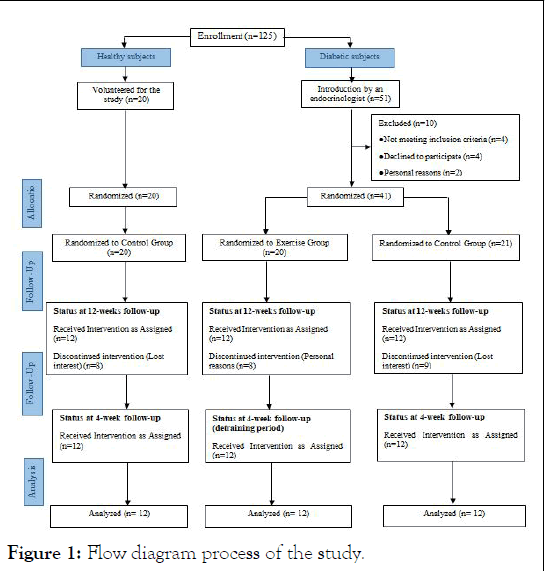
Figure 1: Flow diagram process of the study.
| Variables | Groups | ||
| DT | DC | HC | |
| Age (years) | 15.16 (1.80) | 15.25 (1.76) | 15.08 (1.67) |
| Height (cm) | 159.75 (6.89) | 161.33 (9.46) | 173.91 (11.35) |
| Weight (kg) | 50.41 (4.25) | 50.83 (5.27) | 61.75 (12.87) |
| FBS (mg/dl) | 268.75 (47.78) | 280.58 (58.21) | 92.75 (5.22) |
| HbA1c (%) | 11.14 (2.03) | 10.45 (1.97) | 5.09 (0.33) |
| DD (year) | 7.56 (1.24) | 7.89 (1.22) | - |
| Daily step count (number) | 4563.91 (2201.35) | 4565.08 (2276.67) | 6041.41 (1466.52) |
| SBP (mmHg) | 115.2 (6.1) | 114.3 (5.8) | 110.32 (4.7) |
| DBP (mmHg) | 67.8 (3.5) | 68.4 (3.3) | 63.9 (2.9) |
| HRrest (bpm) | 91.83 (9.74) | 92.83 (11.13) | 78.66 (6.45) |
| SD: Standard Deviation; DT: Diabetes Training Group; DC: Diabetes Control Group; HC: Health Control Group; FBS: Fasting Blood Sugar; HbA1c: Hemoglobin A1c; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; DD: Diabetic Duration; HR: Heart Rate. | |||
Table 1: General characteristics of subjects (mean ± SD).
Electrocardiography
Echocardiography was performed on all participants 3 times (baseline, 12 weeks, and detraining) in the supine, left lateral position using general electric (Mylabseven, Esaote, Italia) system with probe 3 or 5 MHz (multi-frequency transducer) according to the age of subjects. To ensure the reliability of our measurement, we conducted the measurement twice in each step. We only confirmed the results if the difference between the repeated measurements was clinically acceptable. The electrocardiography cable was connected to the ultrasound machine to define and time the cardiac cycle events. The examination was performed by a pediatric cardiologist expert at Cardiovascular Medical Research and Training Center (Farshchian) in accordance with the recommendations of the American Society of Echocardiography. The M-mode rest echocardiographic included standard measurements of dimensions of the Aorta (AO), Left Atrium (LA), Right Ventricular (RV), Left Ventricular Internal Diameter during diastole period (LVIDd), and Left Ventricular Internal Diameter in systolic period (LVIDs), Interventricular Septum (IVS), Left Ventricular Posterior Wall (LVPW), Fractional Shortening (FS), and Ejection Fraction (EF). As well, trans-mitral and trans-tricuspid flows were obtained with pulsed wave Doppler at the leaflet tips; early diastolic inflow velocity (E), velocity during active atrial contraction (A), E to A wave (E/A) ratio, Deceleration Time (DT) were measured, and Myocardial Performance Index (MPI). In order to improve the accuracy of calculations, M-mode echo variables were obtained in terms of Body Surface Area (BSA).
Graded Exercise Test (GXT)
To evaluate aerobic capacity in the participants, a Graded Exercise Test (GXT) was conducted. The test was performed after 4:00 pm to avoid the effect of insulin administration during the morning hours. The participants underwent modified Bruce exercise testing protocol on a treadmill (XSCRIBE; TM65, Mortara, Italian) under controlled laboratory conditions. The modified Bruce protocol is commonly used to evaluate aerobic capacity in children and patients. The target zone for the exercise test was determined based on the ability to achieve to the ability to achieve ≥ 85% (208-(0.7 × age in years)) threshold. VO2peak was calculated using the Bruce equation (Formula 1) and running time during the GXT.
Formula 1: VO2peak formula; T: running time GXT test. 14.8-(1.379 × T)+(0.451 × T2 )-(0.012 × T3)
Exercise training
The exercise program consisted of 12 weeks and 3 days per week of Interval and Resistance Training (IRT) directly supervised by an exercise physiologist. All subjects were prohibited from heavy physical activity 24 hours before the laboratory testing. The IRT training took place in the morning, with awareness of blood glucose levels within the safe range (140 to 250 mg/dL). Each exercise session started with 10-15 minutes of warming up and stretching, followed by 10 to 15 minutes resistance training and 30-55 minutes interval training (running and swimming), and ended with a 10-minute cooling down period. Before running, we did some resistance training to reduce the risk of hypoglycemia during exercise. We determined our One Repetition Maximum (1RM) using Holten's indirect method with weights. We set the weights so that we could do 6 to 12 repetitions, and from this number, we calculated our 1RM using the Holten chart. We started each training session with four different resistance movements, including bodyweight exercises like lunges and pushups, as well as muscle-strengthening workouts such as leg press, bench press, leg extension, seated shoulder press, bicep curl, and tricep dips. For all exercises, we performed three sets of 8 to 12 repetitions and rested for 1.5 to 2 minutes between sets.
Interval training sessions included 3-6 intervals of 5-minute running at an intensity of 50-75% HRR, followed by 4 minutes of recovery at an intensity of 10-20% HRR. We monitored our heart rate during all training sessions using a heart rate monitor (Polar H10, Finland). Additionally, every three weeks, we included a training session in the shallow area of the pool, which consisted of resistance moves of front crawl leg movement and full front crawl swimming intervals with the same time and intensity as the running sessions (sessions 9, 18, 27, 36). To monitor our heart rate during the pool session, we used a waterproof heart rate monitor watch (Beurer PM 58, Germany). We monitored heart rate during exercise to control exercise intensity using Heart Rate Reserve (HRR) methods. During each session, we adjusted our exercise intensity to stay within the prescribed HRR range. Additionally, we increased our exercise volume by 5% each week to prevent us from becoming conditioned to the same routine (Figure 2).
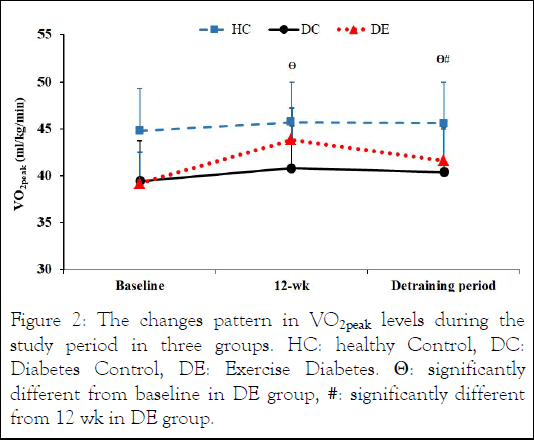
Figure 2: The changes pattern in VO2peak levels during the study period in three groups. HC: healthy Control, DC: Diabetes Control, DE: Exercise Diabetes. Θ: significantly different from baseline in DE group, #: significantly different from 12 wk in DE group.
Laboratory measurements
Glycemic changes were monitored with glycated hemoglobin (A1C) and Fasting Blood Sugar (FBS) measured after 12 hours of overnight fasting at three time points (baseline, after 12 weeks of exercise training, and after 4 additional weeks of detraining) for 3 groups. Serum extracted for laboratory evaluations was obtained from 15 ml blood samples (brachial vein of the right hand) centrifuged at 4 to 8°C with 4000 rpm for 10 minutes. A1C and FBS were analyzed using commercial quality-controlled kits (LDN, Germany) via the enzymatic method by Noble Laboratory in Isfahan, Iran (33) (Figure 3).
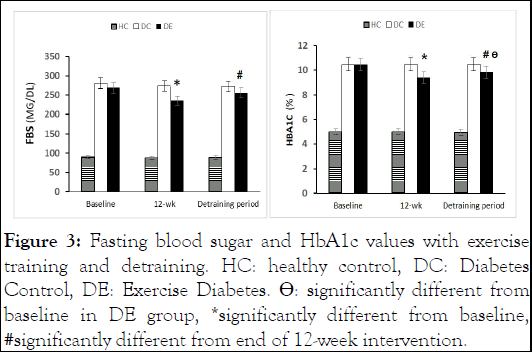
Figure 3: Fasting blood sugar and HbA1c values with exercise training and detraining. HC: healthy control, DC: Diabetes Control, DE: Exercise Diabetes. ÃÃÃÂÂÂÂô: significantly different from baseline in DE group, *significantly different from baseline, #significantly different from end of 12-week intervention.
Statistical analysis
Data normality analysis was determined by the Shapiro–Wilk test. Analysis of variance with repeated measures and Bonferroni's post hoc test were used to compare the differences between and within groups. The difference between the baseline stage and after 12 weeks of intervention was calculated as (Δ1). The difference between the exercise program intervention and the 1 month detraining period was defined as (Δ2), as well the difference between the baseline stage and the detraining period was determined as (Δ3). Data were analyzed using the IBM SPSS Version 26 software. Results presented as mean ± the Standard Deviation (SD) and at level P<0.05. The graphs were drawn using Graph Pad Prism 9.4.1 software, while the formulas were written using Microsoft Equation v. 3.1 (Figure 4).
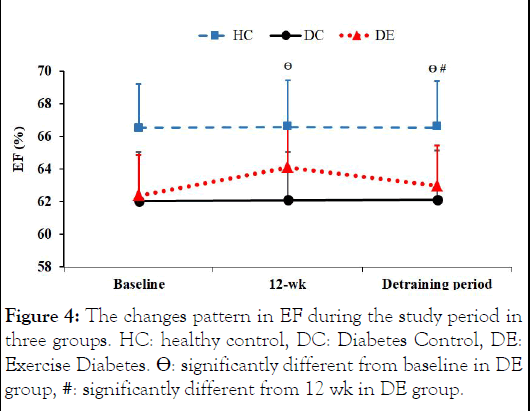
Figure 4: The changes pattern in EF during the study period in three groups. HC: healthy control, DC: Diabetes Control, DE: Exercise Diabetes. Θ: significantly different from baseline in DE group, #: significantly different from 12 wk in DE group.
During the training protocol, under the supervision of an endocrinologist, the injection rate of rapid-acting insulin decreased in the DE group decreased by 56.14%. Table 2 shows the physiological and anthropometric characteristics, glycemic index, and VO2peak values of all the participants across three phases-baseline, 12 weeks, and detraining. The data was analyzed using repeated measures and the Bonferroni post hoc test. There was no significant difference in any physiological, biochemical, or echocardiographic parameters between the DC and DE groups at baseline. A1C and FBS were significantly higher in the T1D groups compared HC group (P<0.05). In contrast, groups with T1D had lower VO2peak compared to HC (P<0.005), although BMI and LVMPI were not significantly different in T1D patients than in healthy adolescent males.
LVIDs, LVPW, and DT tricuspid magnitudes were significantly higher in T1D groups than in HC (P<0.05), whereas FS, EF, Etricuspid, A tricuspid, and E mitral were significantly lower in T1D than HC (P<0.005). AO, LA, RV, LVIDd, IVS, E/A ratio tricuspid, A mitral, E/A ratio, and DT mitral were not significantly different among groups (Table 2).
| Groups/Variables | HC | DC | DE | Observed power | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 wk | Follow-up | Baseline | 12 wk | Follow-up | Baseline | 1 2wk | Follow-up | ||
| BMI (kg/m2) | 20.12 (2.66) | 20.17 (2.56) | 20.35 (2.61) | 19.49 (1.11) | 19.53 (1.01) | 19.47 (1.02) | 19.73 (1.12) | 19.80 (1.22) | 19.79 (1.15) | 0.166 |
| VO2peak (ml/kg/min) | 44.80 bc (4.83) | 45.73 b (3.89) | 45.63 bc (4.28) | 39.43 a (3.40) | 40.83 a (1.98) | 40.39 a (2.26) | 39.20 a (3.32) | 43.86 (3.36) | 41.64 a (3.40) | 0.913 |
| FBS (mg/dl) | 90.75 bc (5.47) | 88.25 bc (5.34) | 89.08 bc (5.69) | 280.58 a (58.21) | 273.91 ac (38.39) | 272.08 a (39.85) | 268.75 a (47.78) | 235.25 ab (45.62) | 255.66 a (45.84) | 0.967 |
| HbA1c (% ) | 5.0 bc (0.30) | 5.0 bc (0.44) | 4.97 bc (0.40) | 10.45 a (1.97) | 10.48 a (1.92) | 10.48 a (1.89) | 10.44 a (2.03) | 9.38 a (1.66) | 9.83 a (1.73) | 0.99 |
| AO (mm/m2) | 14.59 (0.98) | 14.60 (0.91) | 14.61 (0.96) | 15.05 (1.04) | 15.04 (1.09) | 15.08 (1.10) | 15.11 (0.78) | 15.09 (0.77) | 15.10 (0.78) | 0.499 |
| LA (mm/m2) | 16.70 (1.19) | 16.72 (1.19) | 16.72 (1.18) | 17.02 (1.30) | 17.02 (1.33) | 17.03 (1.33) | 17.06 (0.94) | 17.03 (0.94) | 17.05 (0.94) | 0.201 |
| RV (mm/m2) | 10.89 (0.60) | 10.90 (0.61) | 10.90 (0.62) | 10.39 (0.75) | 10.37 (0.76) | 10.36 (0.75) | 10.46 (0.85) | 10.49 (0.87) | 10.49 (0.87) | 0.226 |
| LVIDd (mm/m2) | 27.97 (2.74) | 27.97 (2.72) | 27.96 (2.72) | 29.15 (2.05) | 29.12 (1.99) | 29.13 (2.00) | 29.32 (1.67) | 28.90 (1.65) | 28.91 (1.65) | 0.297 |
| LVIDs (mm/m2) | 16.66 bc (2.40) | 16.81 b (2.54) | 16.88 b (2.51) | 18.75 a (1.89) | 18.78 a (1.92) | 18.77 a (1.92) | 18.90 a (1.37) | 17.94 (1.42) | 18.10 (1.42) | 0.634 |
| IVS (mm/m2) | 4.64 (0.38) | 4.65 (0.37) | 4.65 (0.41) | 4.84 (0.38) | 4.84 (0.38) | 4.85 (0.40) | 4.85 (0.26) | 4.78 (0.24) | 4.79 (0.27) | 0.397 |
| PW (mm/m2) | 4.31 bc (0.50) | 4.30 bc (0.44) | 4.32 bc (0.45) | 4.72 a (0.29) | 4.75 a (0.32) | 4.73 a (0.32) | 4.72 a (0.29) | 4.64 a (0.22) | 4.67 a (0.31) | 0.98 |
| FS (%) | 37.02 bc (0.31) | 37.10 b (0.44) | 37.05 b (0.63) | 36.37 a (0.27) | 36.42 a (0.24) | 36.45 a (0.31) | 36.33 a (0.38) | 36.75 (0.37) | 36.58 (0.48) | 0.986 |
| EF (%) | 66.52 bc (1.01) | 66.56 bc (1.0) | 66.55 bc (1.03) | 62.04 a (1.12) | 62.09 ac (1.03) | 62.12 a (1.0) | 62.38 a (1.60) | 64.08 ab (1.18) | 62.97 a (1.56) | 0.997 |
| E tricuspid (m/sec) | 0.66 bc (0.03) | 0.65 b (0.03) | 0.65 bc (0.04) | 0.61 a (0.01) | 0.61 a (0.01) | 0.61 a (0.02) | 0.61 a (0.02) | 0.62 (0.03) | 0.61 a (0.03) | 0.952 |
| A tricuspid (m/sec) | 0.48 bc (0.03) | 0.47 bc (0.03) | .48 bc (0.03) | .43 a (0.01) | .44 a (0.01) | .44 a (0.02) | .43 a (0.02) | .45 a (0.01) | .44 a (0.02) | 0.975 |
| E/A ratio tricuspid | 1.37 (0.04) | 1.37 (0.04) | 1.37 (0.07) | 1.39 (0.03) | 1.38 (0.04) | 1.39 (0.06) | 1.41 (0.06) | 1.38 (0.05) | 1.40 (0.06) | 0.224 |
| DT tricuspid (ms) | 162.39 bc (1.67) | 162.36 bc (2.22) | 162.17 bc (1.94) | 172.47 a (6.79) | 172.32 a (6.84) | 172.30 a (6.80) | 172.50 a (7.84) | 169.68 a (7.13) | 170.86 a (7.56) | 0.977 |
| E mitral (m/sec) | 0.96 bc (0.03) | 0.96 bc (0.03) | 0.96 bc (0.03) | 0.90 a (0.01) | 0.90 a (0.01) | 0.91 a (0.01) | 0.90 a (0.01) | 0.91 a (0.01) | 0.91 a (0.01) | 0.998 |
| A mitral (m/sec) | 0.54 (0.02) | 0.54 (0.02) | 0.54 (0.02) | 0.52 (0.01) | 0.52 (0.01) | 0.52 (0.01) | 0.52 (0.02) | 0.52 (0.02) | 0.52 (0.02) | 0.615 |
| E/A ratio mitral | 1.77 (0.04) | 1.77 (0.06) | 1.76 (0.07) | 1.73 (0.06) | 1.73 (0.07) | 1.73 (0.07) | 1.72 (0.06) | 1.75 (0.06) | 1.74 (0.06) | 0.226 |
| DT mitral (ms) | 149.21 (1.36) | 149.36 (1.46) | 149.33 (1.52) | 150.66 (1.09) | 150.64 (0.81) | 150.64 (0.81) | 150.67 (1.75) | 150.01 (1.53) | 150.26 (1.68) | 0.609 |
| LVMPI | .269 (0.029) | .265 (0.022) | .264 (0.023) | .25 (0.023) | .256 (0.027) | .257 (0.025) | .252 (0.022) | .268 (0.021) | .263 (0.024) | 1.155 |
| Values are mean ± SD. SD: Standard Deviation. HC: healthy control, DC: diabetes control, DE: exercise diabetes. a: significantly different with HC group, b: significantly different with DC group, c: significantly different with DE group, P<0.05. All values are presented as (Mean ± SD). BMI: Body Mass Index, Vo2peak: Peak oxygen uptake, FBG: Fasting blood glucose, HbA1c (%) : hemoglobin A1C , AO: aorta, LA: left atrium, RV: right ventricle, LVIDd: left ventricular internal dimension during diastole, LVIDs: left ventricular internal dimension during systole, IVS: interventricular septum, PW: posterior wall, FS: fractional shortening, EF: ejection fraction, E: E wave velocity, A: A wave velocity, DT: deceleration time, MPI: myocardial performance index, LV: left ventricle | ||||||||||
Table 2: Subjects’ physiological and echocardiographic characteristics in phases (baseline, 12 weeks, detraining periods).
Comparison between groups in Table 2 showed that after IRT periods, VO2peak and E tricuspid parameters in the DE group are not significantly different from HC and DC. After detraining, these changes returned to baseline (P<0.005). With 12 weeks of IRT, FS and LVIDs parameters in the DE group did not significantly differ from HC and DC groups, nor did they differ after detraining.
After the exercise intervention, FBS was significantly different in the DC group (P<0.005), but not after 1 month weeks of detraining. Other parameters did not change significantly with the intervention or detraining.
There was a significant increase in VO2peak after 12 weeks of IRT and decrease after 1 month of detraining in DE participants (P<0.05). Although, VO2peak values were still considerably higher than the baseline condition.
Correlation analysis
Table 3 displays the Pearson correlation between changes in parameters during the baseline stage, 12 weeks of exercise, and 1 month of detraining. After 12 weeks of IRT intervention (Δ1), there were significant correlations between changes in various measures such as FBS, LA, LVIDd, LVIDs, E tricuspid, A tricuspid, DT tricuspid, A mitral, and DT mitral with both VO2peak and A1C changes (P<0.005). Additionally, changes in FBS, LA, LVIDd, LVIDs, E tricuspid, A tricuspid, and DT tricuspid were found to have a significant correlation with FS changes (P<0.005). Changes in FBS, LA, LVIDd, LVIDs, E tricuspid, A tricuspid, DT tricuspid, E mitral, A mitral, and DT mitral were significantly correlated with EF changes (P<0.005). Furthermore, changes in LA, LVIDd, and A tricuspid were significantly correlated with E/A ratio tricuspid changes, while changes in E mitral and A mitral were significantly correlated with E/A ratio mitral changes (P<0.005). Lastly, changes in LVIDd, LVIDs, E tricuspid, DT tricuspid, and DT mitral after 12 weeks of exercise intervention were found to have a significant correlation with LVMPI changes (P<0.005) (Table 3).
| Parameters | VO2peak (%) (ml/kg/min) | HbA1c (% ) | FS (%) | EF (%) | E/A ratio tricuspid | E/A ratio mitral | LVMPI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | ||
| BMI (kg/m2) | Δ1 | -0.029 | 0.867 | -0.176 | 0.305 | -0.101 | 0.556 | 0.059 | 0.731 | -0.096 | 0.579 | -0.086 | 0.617 | -0.162 | 0.345 |
| Δ2 | 0.022 | 0.898 | -0.077 | 0.656 | 0.09 | 0.601 | -0.019 | 0.913 | -0.261 | 0.124 | 0.105 | 0.544 | 0.192 | 0.262 | |
| Δ3 | 0.02 | 0.907 | -0.096 | 0.578 | -0.308 | 0.067 | -0.078 | 0.649 | 0.015 | 0.929 | 0.128 | 0.458 | -0.315 | 0.061 | |
| FBS (mg/dl) | Δ1 | -0.677 | 0.001* | 0.511 | 0.001* | 0.529 | 0.001* | 0.562 | 0.001* | 0.253 | 0.136 | -0.086 | 0.619 | -0.179 | 0.295 |
| Δ2 | -0.653 | 0.001* | 0.636 | 0.001* | -0.264 | 0.119 | -0.777 | 0.001* | 0.051 | 0.769 | 0.006 | 0.973 | -0.297 | 0.079 | |
| Δ3 | -0.492 | 0.002* | 0.254 | 0.135 | -0.315 | 0.061 | -0.337 | 0.045* | 0.107 | 0.534 | 0.15 | 0.383 | 0.111 | 0.52 | |
| AO (mm) | Δ1 | 0.156 | 0.364 | 0.126 | 0.465 | -0.084 | 0.627 | -0.034 | 0.844 | 0.169 | 0.324 | 0.203 | 0.235 | 0.003 | 0.986 |
| Δ2 | 0.171 | 0.318 | -0.02 | 0.909 | 0.015 | 0.932 | 0.068 | 0.695 | 0.442 | 0.071 | 0.178 | 0.3 | 0.066 | 0.701 | |
| Δ3 | 0.443 | 0.073 | 0.217 | 0.203 | -0.087 | 0.614 | -0.179 | 0.297 | 0.144 | 0.404 | 0.292 | 0.084 | 0.205 | 0.231 | |
| LA (mm) | Δ1 | 0.343 | 0.040* | 0.529 | 0.001* | -0.44 | 0.007* | -0.443 | 0.007* | 0.33 | 0.049* | -0.053 | 0.758 | 0.179 | 0.295 |
| Δ2 | 0.028 | 0.871 | 0.143 | 0.406 | 0.101 | 0.558 | -0.1 | 0.56 | 0.081 | 0.968 | -0.071 | 0.909 | 0.013 | 0.94 | |
| Δ3 | -0.282 | 0.095 | 0.135 | 0.434 | -0.159 | 0.354 | -0.319 | 0.058 | 0.099 | 0.564 | 0.021 | 0.905 | -0.083 | 0.63 | |
| RV (mm) | Δ1 | 0.123 | 0.475 | -0.177 | 0.301 | 0.218 | 0.202 | 0.247 | 0.146 | -0.237 | 0.164 | -0.062 | 0.721 | 0.039 | 0.82 |
| Δ2 | 0.07 | 0.684 | 0.252 | 0.138 | 0.09 | 0.602 | -0.02 | 0.907 | -0.176 | 0.305 | 0.016 | 0.925 | -0.244 | 0.151 | |
| Δ3 | 0.163 | 0.341 | -0.312 | 0.064 | -0.225 | 0.187 | 0.191 | 0.265 | -0.009 | 0.961 | 0.145 | 0.398 | -0.045 | 0.795 | |
| LVIDd (mm) | Δ1 | -0.56 | 0.001* | 0.584 | 0.001* | -0.512 | 0.001* | -0.671 | 0.001* | 0.361 | 0.031* | -0.291 | 0.085 | -0.54 | 0.001* |
| Δ2 | -0.107 | 0.534 | -0.065 | 0.706 | -0.181 | 0.292 | -0.187 | 0.274 | 0.132 | 0.443 | 0.106 | 0.537 | -0.111 | 0.521 | |
| Δ3 | -0.48 | 0.003* | 0.475 | 0.003* | -0.107 | 0.534 | -0.504 | 0.002* | 0.084 | 0.625 | -0.162 | 0.344 | -0.314 | 0.062 | |
| LVIDs (mm) | Δ1 | -0.597 | 0.001* | 0.63 | 0.001* | -0.641 | 0.001* | -0.703 | 0.001* | 0.099 | 0.568 | -0.042 | 0.806 | -0.594 | 0.001* |
| Δ2 | -0.114 | 0.509 | 0.134 | 0.437 | 0.07 | 0.685 | -0.262 | 0.123 | -0.283 | 0.095 | 0.094 | 0.585 | -0.182 | 0.288 | |
| Δ3 | -0.406 | 0.014* | 0.557 | 0.001* | -0.175 | 0.307 | -0.62 | 0.001* | -0.098 | 0.57 | -0.008 | 0.963 | -0.442 | 0.010* | |
| IVS (mm) | Δ1 | -0.162 | 0.346 | 0.044 | 0.799 | 0.033 | 0.849 | -0.197 | 0.248 | 0.026 | 0.878 | -0.207 | 0.225 | -0.23 | 0.177 |
| Δ2 | 0.002 | 0.99 | 0.031 | 0.86 | 0.056 | 0.746 | -0.191 | 0.264 | -0.11 | 0.524 | 0.143 | 0.405 | -0.138 | 0.423 | |
| Δ3 | -0.115 | 0.505 | 0.017 | 0.921 | 0.021 | 0.904 | 0.101 | 0.558 | 0.048 | 0.781 | -0.276 | 0.103 | -0.39 | 0.061 | |
| PW (mm) | Δ1 | -0.241 | 0.157 | 0.229 | 0.179 | -0.099 | 0.567 | -0.276 | 0.103 | 0.226 | 0.185 | 0.072 | 0.677 | 0.013 | 0.942 |
| Δ2 | -0.146 | 0.396 | 0.14 | 0.416 | 0.168 | 0.327 | -0.05 | 0.773 | -0.083 | 0.63 | -0.197 | 0.251 | 0.096 | 0.576 | |
| Δ3 | -0.216 | 0.205 | 0.231 | 0.176 | 0.024 | 0.889 | -0.092 | 0.593 | 0.305 | 0.071 | 0.071 | 0.681 | 0.161 | 0.347 | |
| E tricuspid (m/sec) | Δ1 | 0.561 | 0.001* | -0.418 | 0.011* | 0.376 | 0.024* | 0.614 | 0.001* | 0.1 | 0.56 | 0.182 | 0.287 | 0.468 | 0.004* |
| Δ2 | 0.323 | 0.055 | -0.421 | 0.011* | 0.165 | 0.337 | 0.433 | 0.008* | 0.514 | 0.001* | 0.181 | 0.29 | 0.06 | 0.727 | |
| Δ3 | 0.142 | 0.409 | 0.005 | 0.979 | 0.075 | 0.664 | 0.031 | 0.858 | 0.544 | 0.001* | 0.058 | 0.736 | 0.144 | 0.403 | |
| A tricuspid (m/sec) | Δ1 | 0.591 | 0.001* | -0.505 | 0.002* | 0.607 | 0.011* | 0.701 | 0.001* | -0.743 | 0.001* | 0.237 | 0.163 | 0.321 | 0.056 |
| Δ2 | 0.444 | 0.007* | -0.467 | 0.004* | 0.299 | 0.077 | 0.477 | 0.003* | -0.709 | 0.001* | 0.096 | 0.577 | 0.094 | 0.584 | |
| Δ3 | 0.347 | 0.038* | -0.04 | 0.817 | 0.397 | 0.051 | 0.176 | 0.303 | -0.686 | 0.001* | 0.073 | 0.67 | -0.01 | 0.952 | |
| DT tricuspid (ms) | Δ1 | -0.54 | 0.001* | 0.616 | 0.001* | -0.52 | 0.001* | -0.708 | 0.001* | 0.1 | 0.562 | -0.128 | 0.458 | -0.544 | 0.001* |
| Δ2 | -0.527 | 0.001* | 0.526 | 0.001* | -0.084 | 0.626 | -0.516 | 0.001* | -0.002 | 0.991 | -0.175 | 0.308 | -0.393 | 0.018* | |
| Δ3 | -0.26 | 0.125 | 0.405 | 0.014* | -0.119 | 0.49 | -0.511 | 0.001* | 0.075 | 0.666 | -0.079 | 0.649 | -0.325 | 0.053 | |
| E mitral (m/sec) | Δ1 | 0.266 | 0.177 | -0.182 | 0.288 | 0.203 | 0.235 | 0.399 | 0.016* | -0.234 | 0.169 | 0.751 | 0.001* | 0.297 | 0.078 |
| Δ2 | 0.233 | 0.171 | -0.247 | 0.146 | 0.032 | 0.855 | 0.19 | 0.268 | 0.269 | 0.113 | 0.651 | 0.001* | 0.086 | 0.619 | |
| Δ3 | -0.157 | 0.361 | 0.108 | 0.531 | -0.066 | 0.702 | 0.017 | 0.922 | 0.015 | 0.932 | 0.901 | 0.001* | 0.215 | 0.207 | |
| A mitral (m/sec) | Δ1 | 0.361 | 0.031* | -0.305 | 0.07 | 0.219 | 0.198 | 0.369 | 0.027* | -0.064 | 0.711 | -0.584 | 0.001* | -0.037 | 0.831 |
| Δ2 | 0.271 | 0.11 | -0.162 | 0.345 | 0.132 | 0.443 | 0.266 | 0.117 | 0.161 | 0.347 | -0.522 | 0.001* | -0.175 | 0.306 | |
| Δ3 | 0.003 | 0.984 | 0.063 | 0.715 | 0.092 | 0.595 | 0.192 | 0.262 | 0.044 | 0.798 | -0.137 | 0.426 | 0.076 | 0.658 | |
| DT mitral (ms) | Δ1 | -0.561 | 0.001* | 0.312 | 0.064 | -0.195 | 0.254 | -0.477 | 0.003* | -0.076 | 0.659 | -0.037 | 0.828 | -0.502 | 0.002* |
| Δ2 | -0.422 | 0.010* | 0.221 | 0.196 | 0.035 | 0.841 | -0.351 | 0.036* | -0.018 | 0.916 | 0.134 | 0.437 | 0.142 | 0.41 | |
| Δ3 | -0.44 | 0.007* | 0.142 | 0.41 | 0.074 | 0.667 | -0.05 | 0.771 | -0.033 | 0.85 | -0.01 | 0.953 | -0.422 | 0.010* | |
| RVMPI | Δ1 | -0.038 | 0.827 | 0.25 | 0.142 | 0.005 | 0.978 | -0.007 | 0.969 | 0.141 | 0.413 | 0.007 | 0.968 | -0.046 | 0.789 |
| Δ2 | 0.104 | 0.547 | 0.12 | 0.485 | 0.031 | 0.86 | 0.038 | 0.827 | 0.296 | 0.08 | 0.228 | 0.18 | 0.115 | 0.503 | |
| Δ3 | -0.155 | 0.368 | 0.201 | 0.239 | -0.054 | 0.752 | -0.121 | 0.481 | -0.124 | 0.472 | -0.288 | 0.181 | -0.124 | 0.472 | |
| Note: *: Significant correlation, r: Pearson correlation coefficient, P: P value. Δ1: Changes between baseline and 12 week, Δ2: Changes between 12 wk and 16 wk, Δ1: Changes between baseline and 16 wk. BMI: Body Mass Index; Vo2peak: Peak Oxygen uptake; FBG: Fasting Blood Sugar; HbA1c (% ): Hemoglobin A1C; AO: Aorta; LA: Left Atrium; RV: Right Ventricle; LVIDd: Left Ventricular Internal Dimension During Diastole; LVIDs: Left Ventricular Internal Dimension During Systole; IVS: Interventricular Septum; PW: Posterior Wall; FS: Fractional Shortening; EF: Ejection Fraction; E: E wave velocity; A: A wave velocity; DT: Deceleration Time; MPI: Myocardial Performance Index; LV: Left Ventricle. | |||||||||||||||
Table 3: Correlation between parameter changes in the baseline stage and after 12 weeks exercise and 1 month detraining.
After 1 month of detraining following 12 weeks of IRT intervention (Δ2), there were significant correlations between changes in FBS, A tricuspid, DT tricuspid, and DT mitral with VO2peak changes (P<0.005). Additionally, changes in FBS, E tricuspid, A tricuspid, and DT tricuspid were found to have a significant correlation with A1C changes (P<0.005). Furthermore, changes in FBS, LA, LVIDd, LVIDs, E tricuspid, A tricuspid, DT tricuspid, and DT mitral were significantly correlated with EF changes (P<0.005). Changes in E tricuspid and A tricuspid after 12 weeks of exercise intervention were significantly correlated with E/A ratio tricuspid changes, while changes in E mitral and A mitral after the same duration were significantly correlated with E/A ratio mitral changes (P<0.005). Lastly, changes in DT tricuspid were found to have a significant correlation with LVMPI changes (P<0.005).
Eventually, from the baseline to the end of the detraining period (Δ3), there were significant correlations between changes in various indicators. Specifically, changes in FBS, LVIDd, LVIDs, A tricuspid, and DT mitral were all correlated to changes in VO2peak (P<0.005). Additionally, changes in LVIDd, LVIDs, and DT tricuspid were significantly correlated with changes in A1C levels (P<0.005). Changes in FBS, LVIDd, LVIDs, and DT tricuspid were also found to have a significant correlation with EF changes (P<0.005). Changes in E tricuspid and A tricuspid were linked to changes in E/A ratio tricuspid, while changes in E mitral had a significant correlation with E/A ratio mitral changes (P<0.005). Lastly, changes in LVIDs and DT mitral were significantly correlated with LVMPI changes (P<0.005).
Our investigation examines the effect of an interval aerobic exercise intervention and 1 month of detraining in adolescent males with T1D. Twelve weeks of intervention resulted in a lower glycemic index and higher VO2peak in adolescent males with T1D, and these changes persisted after a month of detraining.
Consistent with previous findings, blood glucose fluctuations are correlated with endothelial disorder and heart function abnormalities, and an inverse relationship with VO2peak has been reported. In contrast to previous investigations, in our study, a poor glycemic index of diabetic subjects was associated with a reduced VO2peak and subclinical cardiac function abnormalities. The participants in this study differ from some previous studies in terms of age, body mass, duration of diabetes, comorbidities and socioeconomic status, exercise intensity, lifestyle, and volume of exercise training and nutritional status. As well, associations between cardiac structure abnormalities, VO2peak and glycemic index in males aged 12 to 18 years with T1D, considering the small number of Intervening variables such as obesity, high-calorie diet, additional cardiovascular risk factors, and less exercise participation It allows a better evaluation of the effect of the exercise intervention.
Although several researchers found no correlation between glycemic measures like FBS and A1C and VO2peak and heart structure, most reported an inverse significant relationship between glycemic index and VO2peak. Scott et al., compared two groups of T1D subjects with intense exercise and moderate and long-term exercises for six weeks (3 times/week). VO2peak and aortic pulse wave velocity variables improved by more than 10% in both groups, but the moderate and long-term exercises group faced a greater drop in glycemic index.
Our study focused on assessment of structure and function of the LV given that variable is more sensitive to inactivity and exercise.
In the baseline of the study, Doppler echocardiography during active atrial contraction and diastolic inflow indicated that our T1D subjects had considerably fewer FS, EF, E mitral, LVMPI, E and A tricuspid variations, compared to the healthy subjects, while significantly higher LVIDs, PW, and DT tricuspid values. However, blood flow velocity measured was not different in comparison to the healthy group. Previous studies have reported alterations in the dimensions and performance of the heart after 6 years and these changes were also found in the youth with T1D in this study.
Chronic hyperglycemia in the early steps of cardiac abnormalities is associated with endothelial dysfunction, filling period and relaxation of ventricles and atria, prolonged time or incomplete filling of the ventricles, and microvascular disease. Cardiac abnormalities can also manifest with pathological myocardial hypertrophy and a reduced LV function in systole and diastole, although these abnormalities may be independent of blood pressure changes and ischemic heart disease.
In the present study, the 12 weeks of IRT resulted in significant improvements in Echo variables (i.e., LVIDd, LVIDs, FS, EF, tricuspid E, tricuspid A, tricuspid DT, and LVMPI variables). However, the effect of such an exercise intervention remained with regard to LVIDd, LVIDs, DT tricuspid, EF, and LVMPI.
Previous studies have shown that the differences in cardiac performance of subjects with and without diabetes are very small in the absence of obvious contractile dysfunction and, therefore, the main determinant of cardiac performance during exercise may be the resting stroke volume and LV contractility state, which may be related to subclinical abnormalities in the structure of the heart.
In prior studies, exercise training programs in individuals with T1D have been associated with increased capillary density and skeletal muscle blood flow, oxidative capacity, increased blood volume, baroreflex sensitivity, heart structure and endothelial performance, a decreased resting cardiopulmonary activity and blood pressure. Also, regular physical activity specifically causes the release of epinephrine and norepinephrine in the circulation system. In the absence of insulin performance, norepinephrine role is the main factor in stimulating glucose uptake by tissues. Likewise, this study found that an improvement of LVIDd and LVIDs and reduction of filling time of left ventricular led to improvement of LV EF and ultimately with favorable cardiac reconstruction following training. This functional improvement may result from a significant increase in LV end-diastolic (Frank- Starling mechanism) filling volume and ventricular and atrial morphological adaptations that support cardiac relaxation during the period. The results of LaMonte et al., are consistent with the observations of this study.
Ravshani et al., and Zhao et al., previously suggested that abnormality in the heart dimensions and performance of T1D people is due to aortic valve disorder and pathological hypertrophy of ventricles and atria at rest situation.
Bezen et al., found a direct relationship between the growth of the LV thickness end-diastolic diameter and the diameter of LVPW with the glycemic index in adults with T1D. However, no significant relationship was found between left ventricular parameters and glycemic index.
Typical heart structure and function treatments it has not been effective and new treatments go on toward drug therapy. According to our data, if heart dysfunction in adolescents with T1D is due to high blood glucose fluctuations, relative cardiac atrophy, or pathological hypertrophy, caused by an inactive lifestyle, common treatment methods may not be effective.
Regular exercise activity under the supervision of an exercise physiologist is a reliable solution in treating the structure and function of the heart, which may be due to direct stimulation of the nervous system and causer mechanisms. Our data revealed that adolescent males with T1D with poor glycemic management have a significant reduction in heart structure and function profile compared to healthy subjects. Also, regular exercise intervention with relatively durable effects can be a probably effective therapeutic method for the control and management of primary structural abnormalities and reduced performance in adolescent males with T1D.
Our T1D cohorts were associated with primary heart structural disorders, decreased performance, and reduced VO2peak compared with healthy subjects. Inactive lifestyle, and pathological and subclinical heart abnormality, were related to a lower VO2peak. These findings suggest that regular interval submaximal aerobic pattern may be a powerful incentive to prescribe exercise prescriptions and reduce the dosage of drugs (under the supervision of an endocrinologist) in people with T1D by lowering the glycemic index and beneficial effects on the structure and performance of the cardiac. It is suggested that in future investigation, a study on the linear or curvilinear regression patterns among LV echocardiography parameters to heart rate indexes was emphasized with a larger sample size of subjects with T1D, an increase in the duration and volume of the interval submaximal aerobic training program, control the daily energy consumption of the subjects.
The current study is a comprehensive study that compares the cardiac structural and functional characteristics of adolescent males with T1D with those of healthy individuals. Then it investigates the effect of 12 weeks of interval submaximal aerobic training on the glycemic index, VO2peak, heart structure and function in this population with T1D. The results of an investigation provide a new non-pharmacological solution for preventing and controlling cardiac problems in youth with T1D. However, when using the results of this study, limitations should be considered including age, gender, sample size, dietary habits, geographic conditions, motivation level, and psychological interventions.
The study was approved by the Research Ethics Committee of Bu-Ali Sina University-Hamedan for humanities studies (Approval ID. IR.BASU.REC.1400.040, IRCT202111031052926N1). Written Informed consent was obtained from all subjects and their parents prior to participation.
Not applicable.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The authors declare no competing interests.
Partial financial support was received from Bu Ali Sina University.
Analysis of the results and supervision of the research was performed by F.N, Collecting data and Supervising the implementation of exercise training was done by H. S, Cardiovascular specialized evaluation and monitoring of the physical condition of the subjects were done by FF and RS.
We thank all participating in the study. In addition, we thank Dr. Sheri Colberg assisted in the final editing of this article.
The authors whose names are listed certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; membership, employment), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Saki H, Nazem F, Fariba F (2025) Impact of Interval and Resistance Training Intervention on Glycemic Index, Aerobic Capacity, Cardiac Structure and Performance in Adolescent Males with Type 1 Diabetes. Biochem Pharmacol. 14:381.
Received: 25-Oct-2023, Manuscript No. BCPC-23-27561; Editor assigned: 27-Oct-2023, Pre QC No. BCPC-23-27561 (PQ); Reviewed: 10-Nov-2023, QC No. BCPC-23-27561; Revised: 07-Jan-2025, Manuscript No. BCPC-23-27561 (R); Published: 14-Jan-2025 , DOI: 10.35248/2167-0501.25.14.381
Copyright: © 2025 Saki H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.