
Cell & Developmental Biology
Open Access
ISSN: 2168-9296

ISSN: 2168-9296
Research Article - (2020)Volume 9, Issue 2
Purpose: The use of pesticides and their potential impact on public health are of great concern. Recent studies on the pathophysiology of neurodegenerative diseases have suggested that chronic exposure to pyrethroids could be associated with alterations in the central auditory pathways. This study was therefore aimed at evaluating the neurotoxicity of pyrethroids on the intracranial auditory relay-centers in adult wistar rats. Methods: Forty adult wistar rats of both sexes with average weight of 180g were assigned into five groups (n=8). Groups A, B, and C were treated with known pyrethroids formulation containing Cyfluthrin, Imiprothrin, and Prallethrin, diluted with olive oil to form 25%, 50% and 75% concentrations. Groups D and E were administered distilled water and olive oil once treatment negative and positive controls, respectively. The treatment lasted for duration of forty days. At the end of the duration, all animals were sacrificed by cervical dislocation, after which the inferior colliculi (IC), medial geniculate bodies (MGB) and cerebellum were harvested for histology and immuno histochemistry using calbindin and palvalbumin antibodies. Results: Histologically, there was slight degeneration of neuronal cells and vacuolated cytoplasm, dispersed distribution of nissl bodies in several cells of MGB and IC of the treated groups compared to the controls. In the cerebellum, there was abnormal proliferation of cells in the purkinje layer with poor distribution of nissl bodies when compared to control groups. Immuno histochemistry further revealed decreased immuno-reactivity of Calbindin2 and Parvalbumin in the treated tissues compared to the control groups. Conclusion: Pyrethroids influences motor coordination and balance of rats, and also the function of the auditory relay-centres.
Pyrethroids; Auditory relay centres; Medial geniculate bodies; Inferior colliculus; Purkinje cells; Chronic study
Pesticides are chemical substances used to eradicate, repel or control certain forms of plant or animal life that are considered to be pests. The major types of pesticides include herbicides, insecticides, fungicides and fumigants. Pesticides vary in their uptake, mode of action, metabolism, toxicity and elimination from the body. People are exposed to pesticides not only in agriculture but also through the contamination of food, air, drinking water and dust.
As synthetic pesticides, they are sometimes formulated with synergistic chemical agents such as piperonyl butoxide and n-octyl bicycloheptene dicarboximide [1]. This enhances their absorption as well as eases their way across the Blood-Brain Barrier (BBB) into the central nervous system [2], and result in nervous tissue damage. Quite a few sites in the brain contain capillaries that lack BBB properties. These sites are found at the mid-sagittal surface of the cerebral ventricles and known as Circum Ventricular Organs (CVOs). The lack of BBB properties at these sites allows easy movement of materials between the blood, Central Nervous System (CNS) extracellular space, and Cerebrospinal to Spinal Fluid (CSF). This causes exposure of CVO organs parenchyma much higher extracellular levels of pesticides (xenobiotics) than other regions of the brain [3,4].
Pyrethroids are divided into Type I and Type II based on the presence or absence of a cyano group in their structure [5]. Deltamethrin (DLM) which is Type II pyrethroids is known to elicit signs of acute poisoning, salivation, hyper-excitability, choreo-athetosis, and seizures in test subjects [6]. In like manner, exposures to certain concentrations of pyrethroids have been observed to produce effects on the nervous system that shows up later in life as a delayed neuropathology [7]. This could be in form of numbness sensations and tingling, weakness and loss of equilibrium, which can occur after a few weeks of exposure to these agents. Furthermore, chronic exposure to pyrethroids was reported to have an influence in the pathologies of the central auditory system [8]. This has also been documented even in humans by an epidemiological study reporting their neurotoxicity after a prolonged occupational exposure [9]. Consequently, pyrethroids pesticides have now been linked to many neurodegenerative disorders that have not been totally studied [7,10].
Millions of people globally are exposed to known neurotoxicants each year, a fact accentuated by recurrent epidemics of neurologic disease [11]. To make matter worse is the incomplete information on many compounds with neurotoxic effects such as pyrethroids commercial formulations. The extent to which neurologic disability may be related to chronic low-level exposures is not known, neither understanding the general effects of environmental contaminants on brain function [12]. Studies on pyrethroids involving the auditory pathways using rats as a model are rare. Although one study has suggested that it could result to hearing loss by breakage or lesions of the intracranial auditory relay centres by degeneration or breakage of the medial geniculate nucleus and inferior collculus [13], it is not clear how this is possible at molecular level where disease onset begin from. Therefore, neurotoxicological evaluation of the intracranial auditory relay centers will help to understand the effect of pyrethroids on hearing and other related neurological disorders. This study was therefore aimed at determining the immunotoxicological effects of pyrethroids pesticide in the intracranial auditory centers of adult Wistar rat.
Animal Care and Ethical Consideration
The present study was approved by the Research and Ethics Committee of the Faculty of Basic Medical Sciences, University of Medical Sciences, Ondo, Ondo State, Nigeria and was carried out in the Department of Human Anatomy, and The Neuro Lab, Federal University of Technology Akure, both in Ondo State Nigeria. All experiments were conducted in accordance with the Guidelines on Ethical Treatment of Experimental Animals as proposed by the European Union directive (Directive 2010/63/EU). Forty 40 adult Wistar rats of both sexes consisting of 20 males and 20 females were obtained from the University of Medical Sciences’ Animal House. Their weights were within the range of 200- 250g. The animals were transferred into iron cages with compartments and were allowed to acclimatize for about 2 weeks under standard and favourable environmental conditions. They were fed on standard rat pellet and water Ad libitum.
Pyrethroids Pesticide (Cyfluthrin, Imiprothrin, Prallethrin)
A very popular pyrethroids formulation known as Baygon multipurpose insecticide which was produced by Johnson Wax Nigeria Limited, Lagos, Nigeria was used in this experiment. The pyrethroids formulation consist of Cyfluthrin, 0.015%; Imiprothrin, 0.05%; Prallethrin, 0.05% with molecular structures given in Figure 1-3 respectively as active ingredients. The insecticide was purchased from a local supermarket close to the university in Ondo City, Nigeria with NAFDAC registration number: D1-1992L. Anti-Calbindin antibody Goat MAb (ab108404) (4°C) produced by Abcam, and Parvalbumin Antibody Rabbit PVALB(5816) (4°C) was purchased from Novus Biologicals, USA, and were used for the immunohistochemistry. Since pyrethroids are lipophilic, Goya olive oil was also purchased from a local supermarket in Ondo city, Nigeria with NAFDAC number L2-1995L and was used as diluent for the pyrethroids formulation.
Figure 1: Molecular structure of Cyfluthrin.
Figure 2: Molecular structure of Imiprothrin.
Figure 3: Molecular structure of Prallethrin.
Experimental Design
The rats were randomly assigned into five groups (A- E) (n=8) and kept in separate compartments. Group A to C were the treatment groups while D and E were oil control and normal control groups respectively. Goya olive oil was used to dilute the pyrethroids formulation into three grades of:25, 50 and 75% concentration (High, Medium and Low). The rats in Group A were treated with High dose pyrethroids (3 ml/kg/body weight of 75%), rats in Group B were treated with Medium dose pyrethroids (3 ml/ kg/body weight of 50%) while those in Group C were treated with Low dose pyrethroids (3 ml/kg/body weight of 25%) of the agent orally, using an improvised plastic cannula once daily, for a duration of 40 days each [14,15]. Group D was given 3 ml/kg of distilled water as placebo while group E received 3 ml/kg/body weight of the diluent.
Method of Sample Collection
On day 41st of the experiment, the rats were reweighed and sacrificed by cervical dislocation. The heads were harvested by a small craniotomy prepared rostral to the lambdoidal suture about 1.5–2 mm lateral to the midline, thereby exposing the posterior aspect of the thalamus. Consequently, the inferior colliculi and MGBs were carefully exposed and dissected out from the already weighed brains (Toledo weighing balance; METTLER TOLEDO, UK) and were quickly fixed in 10% formal saline for immuno histochemical techniques. Sections of about 3–5 μm were cut by a Slee Medical rotary microtome, stained with haematoxylin and eosin and examined under the light microscope [16]. The slides were viewed and captured on an Omax microscope; Santiago USA, 5mp camera in The Neurology Laboratory, Federal University of Technology, Akure. Cut sections were subjected to immunohistochemical procedures using calbindin and palvalbumin antibodies for medial geniculate body, inferior coliculus and purkinje cells of the cerebellum. The slides were subjected to quality control assessment and stored at room temperature prior to photomicrography.
Tissues Processing for Immuno histochemistry
The calbindin and parvalbumin were analyzed using immunohistochemistry. The fixed tissueswere dehydrated using ascending graded series of ethanol. Once cleared, the tissues were infiltrated in molten paraffin wax in the oven at 58°C, then deparafinized and cut at 5 micron, before being mounted on positively charged slides. Antigen retrieval was performed (optimal time is 20- 30 mins) and sections were washed in Tris- Buffered Saline (TBS) for about 2minutes. After this, endogenous peroxidase blocking was carried out using 0.3% hydrogen peroxide in TBS for 10minutes and washed in TBS again. The sections were further blocked in 2.5% normal animal serum (Vector ® -yellow tube in kit) for 20minutes and then incubated in primary antibodies (1/1000, calbindinand 1:200 at room temperature for 2 hours respectively) and washed in TBS for 5 minutes [17]. The resultant sections were incubated in 1mmPRESSTM (peroxidase) Polymer Anti- Goat IgG Reagent, made in horse (Vector®- purple or pink tube in kit) for 30minutes and then washed for 10minutes. Colour was developed on the sections using 3, 3’-diaminobenzidine Peroxidase (HRP) Substrate kit (Vector), followed by rinsing in tap water and counter-staining in haematoxylin. Dehydration was carried out using ascending grades of alcohol, while clearing was by xylene. Mounting and cover-slipping were done using Distyrene Plasticizer Xylene (DPX). The slides were later subjected to quality control assessment and stored at room temperature prior to photomicrography.
Data Analysis
Data generated from the study were presented in bar chart and test of significance was done using One-way Analysis of Variance at 95% (0.05) confidence limit. Homogeneity of variance test was done and Tukey’s HSD test was used to determine the groups responsible for the significant differences. All statistics were done with the aid of the Statistical Package for Social Sciences (SPSS version 21). The stained slides were analyzed for changes across the groups and later captured on an Omax microscope; Santiago USA, 5mp camera in The Neuroscience Laboratory, Federal University of Technology, Akure.
Effect of Pyrethroids Pesticide on Brain Weight
Figure 2 and 4 show effect of pyrethroids on brain weight in rats. Analysis of variance revealed significant differences (P ≤ 0.05) between the mean brain weights of the treated rats (A – C) in a dose- dependent manner when compared to the normal control group. Turkey’s HSD test further showed a significant increase (P ≤ 0.05) in the mean brain weight between the oil and normal control groups (D & E respectively). This infers the possible influence of the Goya olive in the increase of the brain weight. Each bar represents the mean weight of each of the five groups.
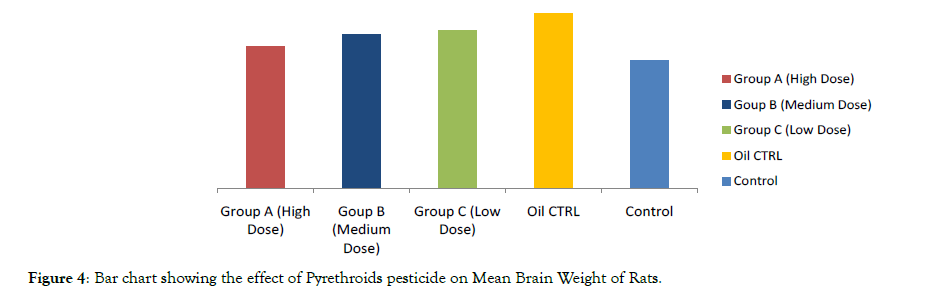
Figure 4: Bar chart showing the effect of Pyrethroids pesticide on Mean Brain Weight of Rats.
Effects of pyrethroids pesticides on the medial geniculate nucleus and inferior colliculus
Figure 5 and 6 shows sections composed of the medial geniculate and inferior colliculus tissues of the rat brain. The histological sections of treated group shows slight degeneration of neuronal cells (slender arrow) in group A having vacuolated cytoplasm when compared to the control group. Group A which was treated with high dose pyrethroids (75%) shows a slight degeneration of neuronal cells, group B and C (medium and low doses pyrethroids) also show a few cells undergoing degeneration while the control groups (D and E) shows the normal architecture of medial geniculate body with intact cytoplasm.
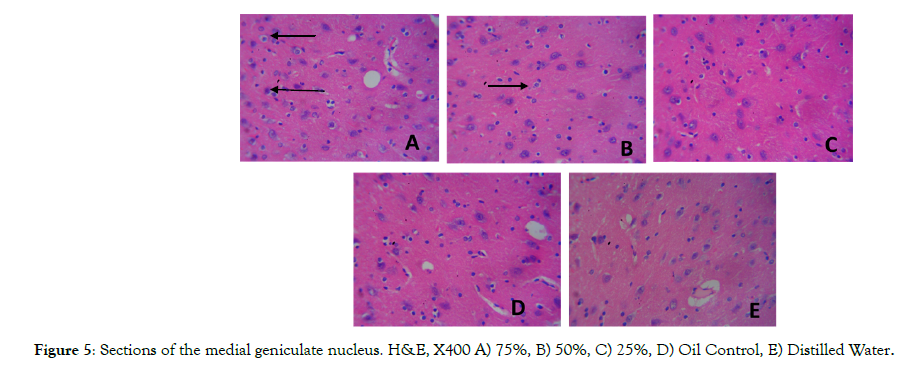
Figure 5: Sections of the medial geniculate nucleus. H&E, X400 A) 75%, B) 50%, C) 25%, D) Oil Control, E) Distilled Water.
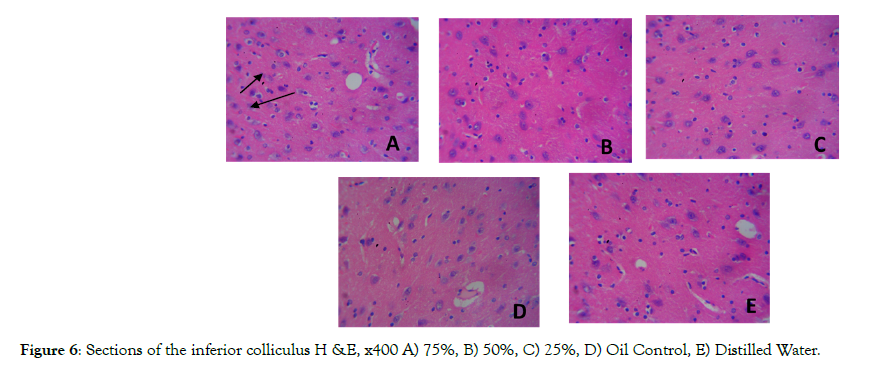
Figure 6: Sections of the inferior colliculus H &E, x400 A) 75%, B) 50%, C) 25%, D) Oil Control, E) Distilled Water.
Effect of Pyrethroids Pesticide on the Purkinje Cells of the Cerebellum (H&E)
Figure 7 shows sections of rats’ inferior colliculus. The histological sections of treated groups A, B, and C (high, medium and low doses pyrethroids respectively) show several normal neuronal cells with normal distribution of nissl bodies (white arrow) and with few cells showing poor distribution of nissl substances (slender arrow) compared to the control groups D and E.
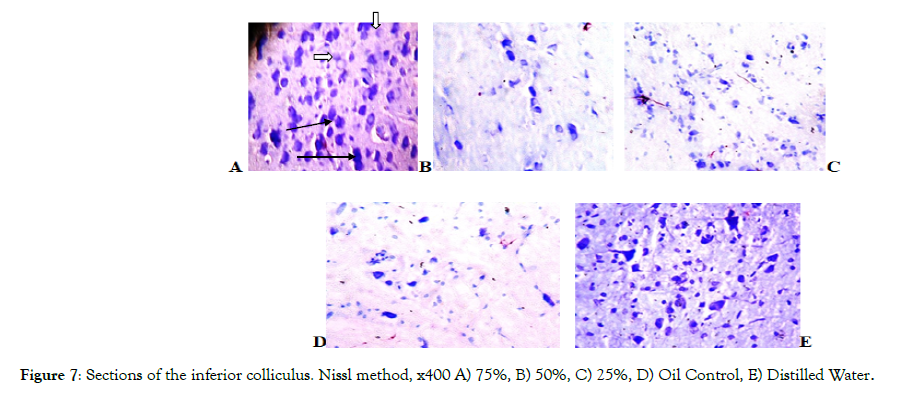
Figure 7: Sections of the inferior colliculus. Nissl method, x400 A) 75%, B) 50%, C) 25%, D) Oil Control, E) Distilled Water.
Figure 8 shows sections of rats’ purkinje cell layer of the cerebellum. The histological sections of treated group show abnormal proliferation of cells in the purkinje cell layer with several of the cells appearing shrunken, when compared to that of the control groups. Group A which was treated with high dose pyrethroids (75%) shows more abnormal proliferation of cells with most of the cells appearing shrunken (see the circled region) while group B and C (medium and low doses pyrethroids also shows few abnormal proliferation of cells with few of the cells shrunken and the control groups (D and E) shows the normal architecture of the purkinje cells of the cerebellum.
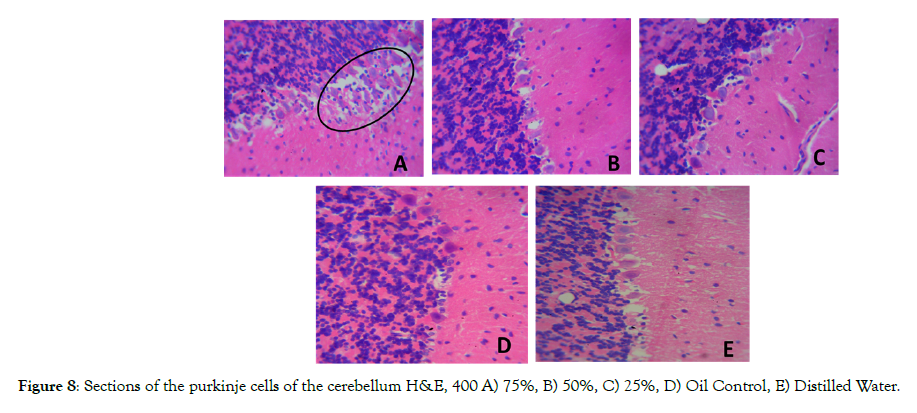
Figure 8: Sections of the purkinje cells of the cerebellum H&E, 400 A) 75%, B) 50%, C) 25%, D) Oil Control, E) Distilled Water.
Figure 9 above shows sections of the purkinje cell layer of the cerebellum. The histological sections of treated group A, B and C shows poor distribution of nissl substances (slender arrows) compared to the control group D and E. The neuronal cells show normal distribution of nissl bodies (white arrow).

Figure 9: Sections of the cerebellum Nissl method x400 A) 75%, B) 50%, C) 25%, D) Oil Control, E) Distilled Water.
Effect of Pyrethroids Pesticides on Calbindin 2 and Palvalbumin Immune Reactivity in the Medial Geniculate Nucleus, Inferior Colliculus and the Purkinje cell layer of the Cerebellum
Figure 10 shows decreased immunoreactivity of calbindin2 (Brownish cell colouration) in the medial geniculate nucleus of group A, B and C compared control group D and E. The immune-reactivity of calbindin2 in group A is the lowest compared to other groups. This shows that pyrethroids decreased the immune-reactivity of calbindin 2.
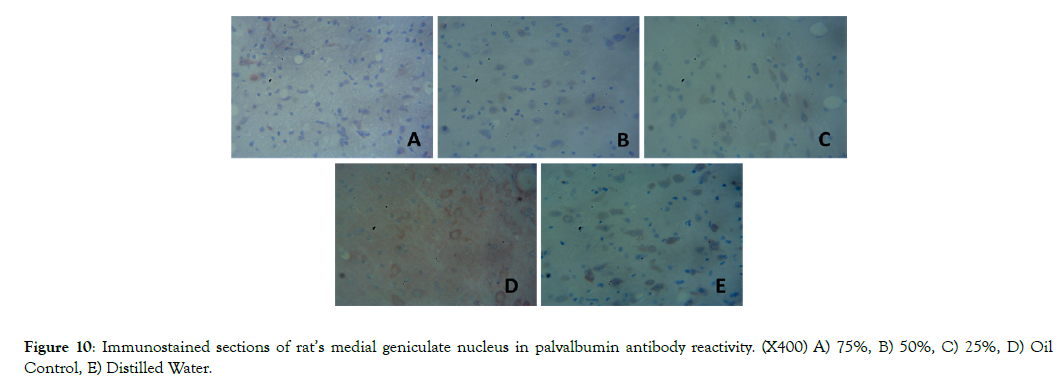
Figure 10: Immunostained sections of rat’s medial geniculate nucleus in palvalbumin antibody reactivity. (X400) A) 75%, B) 50%, C) 25%, D) Oil Control, E) Distilled Water.
Figure 11 shows decreased immunoreactivity of calbindin 2 (Brownish cell colouration) in the inferior colliculusof group A, B and C compared to control groups D and E. The immuno reactivityof calbindin2 is lowest in group A compared to other groups. This shows that pyrethroids decreased the immuno reactivity of calbindin 2.
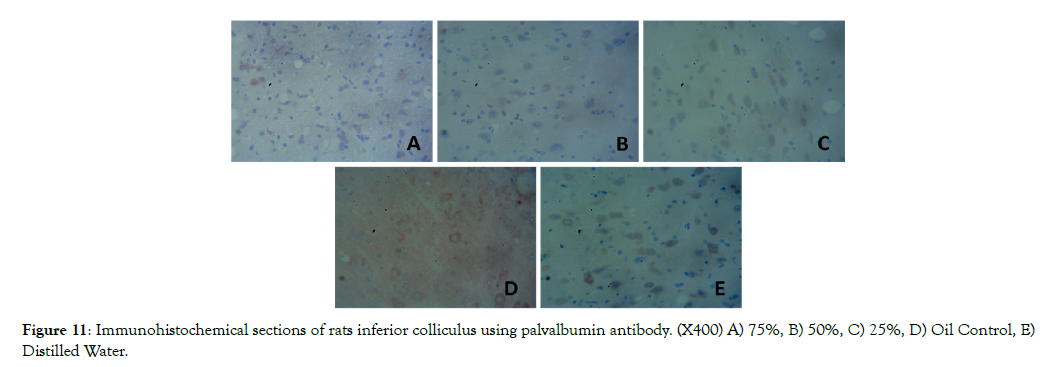
Figure 11: Immunohistochemical sections of rats inferior colliculus using palvalbumin antibody. (X400) A) 75%, B) 50%, C) 25%, D) Oil Control, E) Distilled Water.
Figure 12 shows a decrease in immuno reactivity of calbindin 2 (Brownish cell colouration) in the purkinje cell layer of the cerebellum in group A, B and C compared to the control groups D and E. Immunoreactivity of calbindin 2 is lowest in group A compared to other treatment groups. Therefore this shows that pyrethroids decreased calbindin 2 reactivity.
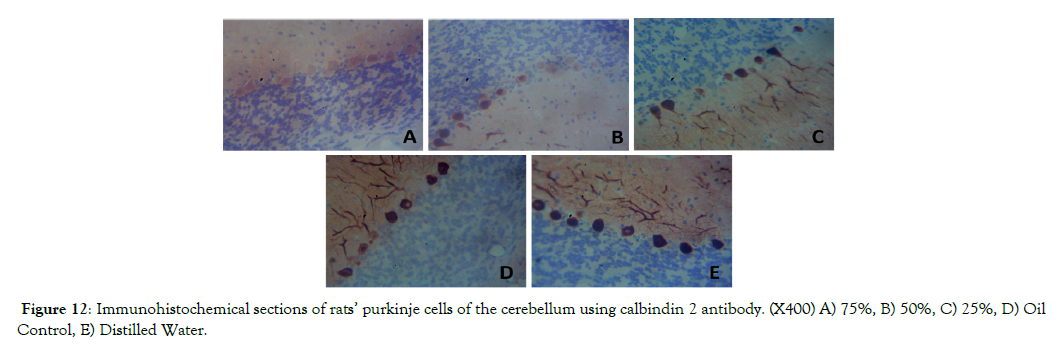
Figure 12: Immunohistochemical sections of rats’ purkinje cells of the cerebellum using calbindin 2 antibody. (X400) A) 75%, B) 50%, C) 25%, D) Oil Control, E) Distilled Water.
Figure 13 shows decrease in parvalbumin immunoreactivity (Brownish cell colouration) in the purkinje cell layer of the cerebellum of the treated groups compared to the control. Immunoreactivity of palvalbumin was highest in the control groups compared to that of the treatment groups (A- C).

Figure 13: Immunohistochemical sections of rats’ purkinje cell layer of the cerebellum using calbindin antibody. (X400) palvalbumin, purkinje cells of the cerebellum A) 75%, B) 50%, C) 25%, D) Oil Control, E) Distilled Water.
Effect of Pyrethroids on Brain Weight
The increase in brain weight observed in this study may have resulted from two major obvious factors. Firstly, the post hoc test revealed the influence of the olive oil which was used as the diluent to have caused a significant increase in brain weight across all the groups, but also, mostly in the oil control group. This suggests that pyrethroids crossed the Blood-Brain Barrier (BBB) due to its lipophilicity at concentrations considered, and perhaps cause damage to endothelial cells of the BBB thereby becoming neurotoxic and presenting with brain oedema. Furthermore, with the oral absorption of this hydrophobic compound, and easy access of the BBB, it entered into the CNS to cause intramyelinic oedema, splitting the intraperiod line of myelin in both the CNS and the PNS [12]. Consequently, this led to formation of vacuoles, and subsequently creating a “spongiosis” of the brain [18] just as was demonstrated in our H& E techniques. Experimental studies with erythrocyte membranes have shown that some neurotoxicant binds tightly to cell membranes, resulting in the loss of ion gradients across the membrane [19]. This loss of the ability to exclude ions from between the layers of myelin leads to water and ion entry, which separates the myelin layers as “oedema.”Additionally, intramyelinic oedema is reversible in the early stages, but with increasing exposure, segmental demyelination may set in and even neuronal death.
This study revealed that pyrethroids pesticide is associated with neuronal cells degeneration and vacuolated cytoplasm corroborating previous studies that exposure of the auditory system to pyrethroids could cause neurodegeneration and affects the auditory relay centers [8]. Certainly, this suggests that highfrequency tonotopic components of auditory pathways afferent to the IC are essentially inactivated, whereas those supporting lower frequencies are not. This imbalance of afferent input has a number of important consequences falling under the rubric hearing-loss– induced (HLI) plasticity and subsequently to sensor neural hearing loss [20,21].
Although, several studies have reported a neurotoxic effect of pesticides [15 ,22 - 24], questions are being raised on the likely risk of the auditory part of the brain to be compromised. Hearing loss is commonly associated with aging, noise exposure, and head trauma, but a growing body of evidences also links hearing loss to chemical exposure. Hearing loss imposes many burdens on workers, including communication difficulties, possible job loss, and stigma. Hearing loss may also increase risk of occupational injury because of inability to hear warning signals or shouts [25].
Similarly, using Nissl stain to identify cyto-architecture of the inferior colliculus and purkinje cells of the cerebellum; there was poor distribution of nissl bodies (chromatolysis) in the treatment groupscompared to the control group showing the demand for protein synthesis [26]. Nissl bodies have been observed to show changes in several physiological and pathological conditions and may dissolve and disappear. Chromatolysis has been linked to several neurodegenerative diseases [27-30], thereby confirming the plausible neurotoxicity of this agent in the auditory centres.
We also evaluated with immunohistochemical techniques the effect that pyrethroids pesticide has on Parvalbumin and Calbindinexpressing system of neurons in the higher structures of the central auditory pathway. Notably, in the inferior colliculus (IC), medial geniculate body (MGB), thalamus and cerebellum of adult Wistar rats. The study revealed decreased immunoreactivity of calbindin 2 antibody in the treated groups compared to the control groups. Calbindin2 (Calretinin) which is a calcium-binding protein is involved in calcium signaling [31]. Its protein is encoded by the CALB2 gene in humans and encodes an intracellular calciumbinding protein belonging to the troponin C superfamily and plays role in diverse cellular functions, including message targeting and intracellular calcium buffering ("Entrez Gene: calbindin 2"). For proper functioning of these processes, the concentration of intracellular free calcium must be maintained within an ideal range. Concentrations outside this ideal range often pose a harmful, if not fatal, effect on neurons [32]. High intracellular calcium has been observed to be toxic to cells and alterations in calcium homeostasis and is associated with changes in calciumbinding proteins, which confine free Ca2+ [33]. Contrarily, decrease in calbindin 2 immunoreactive cells has been observed in deaf mice [34]. Bearing in mind the neuro-protective role of calbindin 2, decrease in its immuneoreactivity could be suggesting an increased susceptibility to Ca2+-dependent pathologies in the auditory system. Our finding is similar to that of a previous study [35] that reported a decrease in immuneoreactivity of calbindin 2 antibody and further suggested decreased concentration of calcium in the neuronal cells, as calcium plays a key role as an intracellular mediator of various physiological processes in nerve cells, including their development, growth, transmitter release, Trans-membrane signaling, and synaptic plasticity [36]. Since calcium binding proteins function to protect neurons from calcium-mediated toxic injury, and also plays a role in diverse cellular functions, a decrease in calbindin 2 immunoreactivity in the IC and MGN will cause neuronal degeneration as calbindin 2might have lost its protective function thereby lowering their activity [37].
Furthermore, this study revealed that there was a decreased immunoreactivity of palvalbumin antibody in the treated group compared to the control group in the purkinje cells layer. Parvalbumin (PV), a calcium-binding albumin protein with low molecular weight is expressed by GABAergic interneurons in the nervous system, especially the reticular thalamus [38]. In the cerebellum, it is highly expressed by Purkinje cells and molecular layer interneurons [39]. In the auditory pathway, PV has been found to be mostly expressed by neurons in the ventro-lateral part of the inferior collicus, and also well expressed in the thalamus and medial geniculate nucleus [40]. Calcium binding proteins like PV play a role in many physiological processes, including cell-cycle regulation, second messenger production, muscle contraction, organization of microtubules and phototransduction [41]. Decrease of the PV in the IC as observed in this study is linked to loss of innervation of the GABAergic neurons as reported in previous studies [39,41]. Moreover, the loss of PV in neurons as well as neuropil has been reported in other parts of the brain as well as IC, leading to substantial deterioration of hearing function (Ouda et al., 2008). Besides, studies of autism spectrum disease (ASD) using animal models [42] revealed the number of PVimmunoreactive (PV+) cells to be decreased, and this was also similar to findings observed in post mortem brains of ASD patients [43]. The decrease of PV+ neurons was presumed to be due to partial loss of the PV neurons. Additionally, PV+ interneurons in auditory cortex was observed to show extra intensely modulated responses to both gaps in noise and bursts of noise, thereby suggesting that they are optimized for the rapid detection of stimulus transients [44].
In conclusion, this study revealed oral administration of pyrethroids pesticide at different doses for forty days resulted in significant histomorph ological changes,decreased palvabumin and calbidin-2 immuno reactivity in the inferior colliculus and medial geniculate nucleus, as well as the cerebella tissues.
The authors wish to acknowledge Raheem Olamide, University of Ibadan, Ibadan, Oyo State, Nigeria and Dr. Ijomone Mamus, Neurology Laboratory, Federal University of Technology, Akure, Ondo State, Nigeria for technical support.
The datasets used and analyzed during the current study are available from the corresponding author on request.
There is no conflict of interest disclosed in this study.
The authors ensure that the manuscript is reported clearly, truthfully and accurately with no omission of any important aspect.
This work was funded only by the authors. This work did not receive any specific grants or funds from funding agencies in the public, commercial or not- for- profit sectors.
IKA: conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, Resources, supervision, validation, roles/writing- original draft, writing review and editing visualization.
AAA: data curation, formal analysis, funding acquisition, methodology, project administration, Resources, roles/writingoriginal draft, visualization, investigation.
TTW: methodology, project administration, Resources, roles/ writing- original draft, visualization, investigation
OO: methodology, project administration, Resources, roles/ writing- original draft, visualization, investigation
All experiments were conducted in accordance with the Guidelines on Ethical Treatment of Experimental Animals as proposed by the European Union directive (Directive 2010/63/EU).
Citation: Kingsley A. Iteire, Adejumo A. Adenike, Tebamifor T. Witness, Onoriode Oyiborhoro (2020) Immunotoxicological Investigation of the Intracranial auditory centers in pyrethroids-formulation treated Wistar rat. Cell Dev Biol 2020; 9:202. doi: :10.24105/2168- 9296.2020.9.202
Received: 22-Dec-2019 Accepted: 18-May-2020 Published: 25-May-2020 , DOI: 10.35248/2168-9296.20.9.202
Copyright: ©2020 Iteire K A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This work was funded only by the authors. This work did not receive any specific grants or funds from funding agencies in the public, commercial or not- for- profit sectors.