Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Case Report - (2022)Volume 13, Issue 8
Background: Hypertriglyceridaemia (HTG) is the most common form of dyslipidaemia. It is rare for patients with high levels of Triglycerides (TGs) to not suffer from Acute Pancreatitis (AP).
Case presentation: We report a 42-year-old male who came in for a physical examination for job entry. His blood lipids showed the following: TGs were significantly increased at 3101 mg/dl, Total Cholesterol (TC) was slightly increased, and High-Density Lipoprotein Cholesterol (HDL-C) was decreased.
Results: There were no changes in amylase, fasting serum glucose, carotid color ultrasound, or Electrocardiogram (ECG). The patient consumed a healthy diet, engaged in physical activity and used fenofibrate tablets, and his blood lipids significantly improved.
Conclusion: Dyslipidaemia is one of the risk factors for Atherosclerotic Cardiovascular Disease (ASCVD). HTG can cause Acute Pancreatitis (AP) and death, so it is very important to control blood lipids. Comprehensive treatment of HTG is effective. Antibody treatment of Hyperlipidaemia (HPL) has broad prospects.
Hypertriglyceridaemia; Triglycerides; Atherosclerotic cardiovascular disease; Cardiovascular disease
HTG: Hypertriglyceridaemia; ASCVD: Atherosclerotic Cardiovascular Disease; CVD: Cardio Vascular Disease; TGs: Triglycerides; TC: Total Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol; ECG: Electrocardiogram; LDL-C: Low-Density Lipoprotein Cholesterol; HPL: Hyperlipidaemia; BMI: Body Mass Index; CCTA: Coronary Computed Tomography Angiography; CHD: Coronary Heart Disease; TPE: Therapeutic Plasmapheresis; HLAP: Hyperlipidaemic Acute Pancreatitis; AP: Acute Pancreatitis; DM: Diabetes Mellitus; SPPARMα: Selective Peroxisome Proliferator-Activated Receptor α Modulator; ASGR1: Asialoglycoprotein Receptor 1; ABCG5: ATP-binding Cassette, Subfamily G (WHITE), Member 5 (sterolin 2); ABCG8: ATP-binding Cassette, Subfamily G (WHITE), Member 8 (sterolin 2); PARM: Proliferator-activated Receptor α Modulator; FGF 21: Fibroblast Growth Factor 21.
This is a rare case of a patient who was diagnosed with mixed Hyperlipidaemia (HPL) with a Triglyceride (TG) level of 35.0 mmol/L (1 mmol/L=88.6 mg/dl) without any discomfort. Hypertriglyceridaemia (HTG) can be divided into primary and secondary types. Primary HTG is mainly genetically related, mainly including familial hypertriglyceridemia, familial abnormalities β-Lipoproteinemia and familial mixed dyslipidemia. Secondary HTG is related to many factors, such as excessive drinking, endocrine disease, kidney disease, use of certain drugs (such as glucocorticoids, diuretics, estrogen β-Receptor blockers, etc.), and other systemic diseases. At present, the most common secondary causes are unreasonable diet, metabolic syndrome and lack of exercise. HTG can cause Acute Pancreatitis (AP), and TG levels are significantly correlated with arteriosclerosis. A large meta-analysis (including 61 studies and 330566 patients) showed that for every 1 mmol/L (88 mg/ dl) increase in TG, the risk of Atherosclerotic Cardiovascular Disease (ASCVD) increased by 22% [1], so early comprehensive intervention is crucial. During the past decades, drugs therapies have achieved some results in HTG. Recently, what’s the most exciting is that neutralizing antibodies against ASGR1 have been found, which can effectively promote cholesterol efflux to lower cholesterol levels. It’s believed that better and newer drugs used for HTG will be developed.
A 42-year-old male participated in a physical examination for job entry at 8:30 a.m. on June 30, 2022. On physical examination, the patient’s body temperature was 36.2°C, pulse rate was 56 beats/min, respiratory rate was 18 breaths/min, blood pressure was 131/81 mmHg, weight was 72.7 kg, height was 1.675 metres, and Body Mass Index (BMI) was 25.91. No xanthomas were found on the skin or tendons. The patient had a clear mind. The cardiac boundary was normal, with a heart rate of 56 beats/min. The heart rhythm was uniform, and there was no murmur in the auscultation area of each valve. The abdomen was flat and soft, without tenderness or rebound pain, and the bowel sound was normal. No other unusual findings were present on his physical examination. He did not have any discomfort. Physical examination results showed no detectable changes in routine blood examination, routine urine test, fasting blood glucose, liver and kidney function, chest X-ray or ECG but marked elevations in the Triglyceride (TG) level, which was 35.00 mmol/L (reference range, 0.57 to 2.26 mmol/L) leading to a “milky” appearance (Table 1); a Total Cholesterol (TC) level of 6.47 mmol/L (reference range, 3.6 to 6 mmol/L); a High- Density Lipoprotein Cholesterol (HDL-C) level of 0.82 mmol/L (reference range, 0.9 to 2.5 mmol/L); and a Low-Density Lipoprotein Cholesterol (LDL-C) level of 1.76 mmol/L (reference range, ≤ 3.34 mmol/L). The patient stated that he usually ate a high-fat diet and that his father suffered from Coronary Heart Disease (CHD). He denied the use of any tobacco or alcohol and did not have other known chronic diseases. There were no similar diseases or any other genetic history of disease other than CHD in his family (Figure 1).
| Biochemical Examination Report of The Second Affiliated Hospital of Guilin Medical University | ||||
|---|---|---|---|---|
| Sample No.: 206 | ||||
| Serial Number | Item name | Result | Reference Range | Unit |
| 1 | *Total Protein | 70.9 | 65-85 | g/L |
| 2 | *Albumin (AI) | 46.4 | 40-55 | g/L |
| 3 | Globulin (GI) | 24.5 | 20-40 | g/L |
| 4 | AI/GI Ratio | 1.89 | 1.1-2.5 | - |
| 5 | Glutamic Ryruvic Transaminas (ALT) | 10 | 9-50 | U/L |
| 6 | *Glutamic Oxaloacetic Transaminase (AST) | 41.2↑ | 15-40 | U/L |
| 7 | AST/ALT Ratio | 4.12 | - | - |
| 8 | *Total Bilirubin | 30.40↑ | 2-20 | umol/L |
| 9 | Direct Bilirubin | 12.00↑ | 0.01-10 | umol/L |
| 10 | Indirect Bilirubin | 18.40↑ | 1.71-12 | umol/L |
| 11 | *Glutamyl Transpeptidase | 79.0↑ | 10-60 | U/L |
| 12 | *Alkaline Phosphatase | 85 | 45-125 | U/L |
| 13 | *Urea Ritrogen | 5 | 3.1-8 | mmol/L |
| 14 | *Creatinine | 109↑ | 57-97 | umol/L |
| 15 | *Glucose | 4.9 | 3.9-6.1 | mmol/L |
| 16 | *Uric Acid | 344 | 208-428 | umol/L |
| 17 | *Total Cholesterol | 6.47↑ | 3.6-6 | mmol/L |
| 18 | *Triglycerides | 35.00↑ | 0.57-2.26 | mmol/L |
| 19 | High-Density Lipoprotein Cholesterol | 0.82↓ | 0.9-2.5 | mmol/L |
| 20 | Low-Density Lipoprotein Cholesterol | 1.76↑ | ≤ 3.34 | mmol/L |
Name:  Medical Record No:
Medical Record No: Bed Number: Patient Type: Physical Examination Gender : male Department: Department of Physical Examination Specimen: Serum Collection Date: 2022-06-30 8:54:43 Age: 42 y Applying Doctor:
Bed Number: Patient Type: Physical Examination Gender : male Department: Department of Physical Examination Specimen: Serum Collection Date: 2022-06-30 8:54:43 Age: 42 y Applying Doctor:  Inspection Purpose: Liver Function, Kidney Function, Blood Lipids Remarks: Muddy (Chylomicrons)
Inspection Purpose: Liver Function, Kidney Function, Blood Lipids Remarks: Muddy (Chylomicrons)
Note: the “*” mark is the mutual recognition project of Guangxi results. This report is only responsible for this sample. The report is for clinican reference only. If you have any queries, please contact us in time. ↑: More than the normal range; ↓: Less than the normal range.
Testing instrument: Cobas c702
Receiving Date: 2022-06-30 10:20:03 Report Date: 2022-06-30 11:37:08 Operator:  Collator:
Collator:
Table 1: (The English report of the patient): Blood lipids showed the following: TGs were significantly elevated at 35.00 mmol/L, Total Cholesterol (TC) was slightly elevated, and the High-Density Lipoprotein Cholesterol (HDL-C) was low.
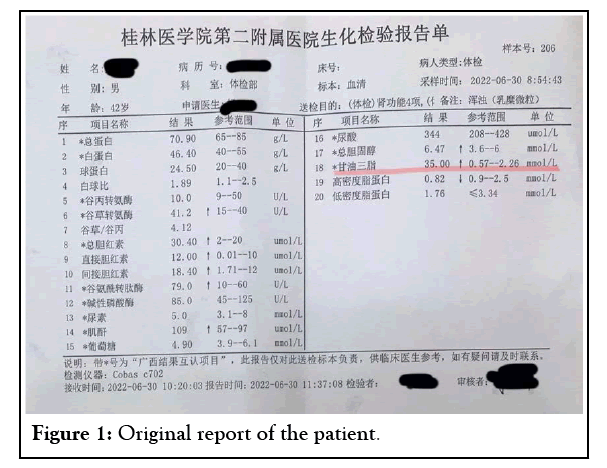
Figure 1: Original report of the patient.
Preliminary diagnosis
The patient was diagnosed with mixed HPL and overweight. As his TG level was severely elevated, he was immediately transferred to the hospital to undergo amylase and other tests. Amylase, thyroid function, glycosylated haemoglobin and carotid colour ultrasound were normal. Colour ultrasound of the liver, gallbladder, pancreas and spleen showed fatty liver. The patient refused genetic testing for blood lipids, blood flow detection, fundus examination and Coronary Computed Tomography Angiography (CCTA).
Final diagnosis
The patient was diagnosed with primary mixed HPL, fatty liver and overweight. He was not hospitalized and did not receive Therapeutic Plasmapheresis (TPE), but he received outpatient treatment and monitoring of blood lipids, myocardial enzymes, liver function, etc. The patient was advised to consume a healthy diet and engage in physical activity (Table 2). He was advised to follow a vegetarian diet for 7 days and to go to the hospital immediately if abdominal pain occurred. He was treated with fenofibrate (0.1 g, tid). After 6 days, re-examination of blood lipids, myocardial enzymes, and liver and kidney function indicated normal levels; however, TGs had not been reduced to an appropriate level, and HDL-C was 0.92 mmol/L. The patient was advised to continue taking fenofibrate. He asked for a consultation with the Department of Nutrition. After 7 days, it was recommended that the intake of fat should not exceed 20%-30% of the patient’s total energy intake. Fat intake should come mainly from foods rich in n-3 polyunsaturated fatty acids (such as deep-sea fish and vegetable oil). The proportion of each macronutrient should be carefully chosen. It was suggested that daily carbohydrate intake should account for 50%-65% of total energy intake, and carbohydrate intake should mainly come from cereals, potatoes and whole grains. The total energy intake should be controlled while meeting the daily essential nutritional needs. The patient should do at least 30 minutes a day of moderate-intensity aerobic physical activity. The patient was advised to control his weight and maintain a healthy weight (BMI 18.5 23.9 kg/m2).
| Dietary Recommendations for Reducing Triglycerides | |
|---|---|
| Elements | Recommendations |
| Limit dietary components to increase TGs | |
| Saturated fatty acids | less than 7% of total energy |
| Dietary cholesterol | ﹤300 mg/d |
| Increase dietary components to increase TGs | |
| Plant slerols | 2-3 g/d |
| Soluble dietary fiber | 10-25 g/d |
| Totol energy | Adjust to reduce weight or ideal weight |
| Physical activity | Keep moderate intensity exercise and burn at least 200 kcal calories every day |
Table 2: Advice on diet and physical activity.
The patient’s TGs showed significant elevation, while LDL-C was normal (1.76 mmol/L). Unfortunately, the patient refused genetic testing for blood lipids. Therefore, the diagnosis of familial HTG or familial HPL could not be excluded. The patient was very lucky that he did not suffer from Hyperlipidaemic Acute Pancreatitis (HLAP), because his TG level was very high. There is a 5% possibility of developing AP if TG levels exceed 11.3 mmol/L, and this possibility rises to 10%-20% if TG levels are over 22.6 mmol/L [2].
Many studies have shown that TG elevation is closely related to an increased risk of Cardiovascular Disease (CVD) and is a risk factor for CVD. Studies have confirmed that in the subgroup with HTG (TG ≥ 2.3 mmol/L) and low HDL-C (HDL-C ≤ 0.88 mmol/L), reducing the level of TGs can effectively reduce the occurrence of cardiovascular events [3-5]. This is the residual cardiovascular residual risk and refers to the risk of patients experiencing macro- and microvascular events even after standard treatment guided by current clinical evidence (including evidence-based risk factors such as an unhealthy lifestyle, hypercholesterolaemia, hypertension, hyperglycaemia, obesity, etc.). Residual cardiovascular risk is related to many factors. The most common one is dyslipidaemia characterized by high TG and low HDL-C, that is, lipid-related cardiovascular residual risk. Therefore, the patient must continue to receive comprehensive treatment to decrease blood lipids to an appropriate level according to the risk assessment of ASCVD. In addition, many systematic reviews have shown that the control of TGs in patients with type 2 diabetes not only has an effect on ASCVD but can also delay or treat microvascular complications [6-8].
Fasting HTG is commonly associated with insulin resistance and frequently occurs in individuals with type 2 Diabetes Mellitus (DM) [9]. Charoensri et al. found that the fasting TG level is an independent risk factor for the development of incident DM over a 10-year period [8]. The key to the treatment of HTG is to reduce the level of TGs and maintain an appropriate level of blood lipids. Comprehensive treatment of HTG is effective. The treatment includes lifestyle changes, lipid-lowering drug therapy, antibody treatment and TPE.
A healthy lifestyle includes a healthy diet and physical activity. The total energy intake should be controlled while meeting daily essential nutritional needs. A healthy dietary pattern consists of nutrient-dense foods and beverages across all food groups in recommended amounts and within calorie limits. The proportion of each nutrient within the diet should be carefully selected. Nutrient-dense foods that provide vitamins, minerals, and other health-promoting components should be encouraged, while limiting foods and beverages that are higher in added sugars, saturated fat, and sodium, as well as alcoholic beverages [10]. Individuals should also quit smoking and should aim to reduce their weight and maintain a healthy weight (BMI 18.5 24.9 kg/m2). The US Department of Health and Human Services recommends that adults engage in at least 150 minutes to 300 minutes a week of moderate-intensity aerobic physical activity, 75 minutes to 150 minutes a week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate and vigorous-intensity aerobic activity. They should also perform muscle-strengthening activities 2 or more days a week. Older adults should engage in multicomponent physical activity that includes balance training as well as aerobic and muscle-strengthening activities [11].
The drugs used for HTG include fibrate, pemafibrate, highpurity medicinal fish oil, and nicotinic acid. Fibrates have been shown to markedly reduce TG levels by 50% or more, indicating their efficacy and encouraging their inclusion in the treatment protocol [12]. Kayıkçıoğlu et al. found that patients were treated with fenofibrate and less frequently gemfibrozile (14 patients) at different doses according to their TG level and disease severity. No rhabdomyolysis or myopathy was seen during the 11-year follow-up. As a first-line pharmacotherapy, long-term fibrate use is effective and safe [13]. Many clinical studies have confirmed that fenofibrate combined with statins is also safe. In the FIELD study, approximately 900 patients were treated with fenofibrate combined with statins, and no rhabdomyolysis occurred during the 5-year follow-up [14]. It is noteworthy that the application of antibiotics such as xylenoxyheptanic acid or clarithromycin in patients using simvastatin may cause rhabdomyolysis [15].
Many patients with HTG have abnormal glucose metabolism and liver function. An increased risk of cardiovascular events is associated not only with dyslipidaemia but also with abnormalities in glucose metabolism and liver function [16]. Pemafibrate is a Selective Peroxisome Proliferator-Activated Receptor α Modulator (SPPARMα) used in patients with HTG. Pemafibrate can improve glucose metabolism and liver function and increase Fibroblast Growth Factor (FGF) 21 without increasing the adverse event risk [16].
The main component of high-purity fish oil is n-3 fatty acids. The study of Bhatt, et al. showed that high-dose high-purity fish oil (total daily dose, 4 g) can significantly reduce TGs and reduce the occurrence of adverse cardiovascular events in patients with ASCVD [17]. Nicotinic acid can reduce TG levels by 35%. It can commonly be used as an adjunct to fibrate monotherapy in those at high risk for pancreatitis [18].
Currently, no drug lowers cholesterol by directly promoting cholesterol excretion. Human gene studies have revealed that loss-of-function Asialoglycoprotein Receptor 1 (ASGR1) variants are associated with low cholesterol and a reduced risk of CVD. ASGR1 is exclusively expressed in the liver and mediates internalization and lysosomal degradation of blood asialoglycoproteins. Wang et al. found that inhibition of ASGR1 led to increased expression of ATP-Binding Cassette, Subfamily G (WHITE), Member 5 (sterolin 2) (ABCG5) and ATP-Binding Cassette, Subfamily G (WHITE), Member 8 (sterolin 2) (ABCG8), which discharge cholesterol into bile and through faeces, thus greatly reducing the level of lipids in the blood and liver; this had an obvious curative effect on atherosclerosis and fatty liver. Neutralizing antibodies against ASGR1, which can effectively promote cholesterol efflux and have good synergistic lipid-lowering effects with statins and ezetimibe, have also been developed [19]. Antibody treatment of HPL has broad prospects.
HTP types I, IV, and V mostly cause AP. HTG has become the third most common cause of AP. Its reported incidence is 2%-4% [20-21]. Excess TGs are hydrolysed by lipase from pancreatic acinar cells to produce Free Fatty Acids (FFAs). These high concentrations of FFAs trigger an inflammatory reaction, releasing intracellular calcium and causing acinar necrosis. High levels of FFAs aggregate into micelles with detergent-like properties, causing ischaemia, triggering acidosis, and activating trypsinogen to form active trypsin, resulting in pancreatic autodigestion [22]. The association between HPL and AP was first described by Speck in 1865 [23]. TPE as a treatment for HLAP was first described in 1978 [24].
It is believed that the risk and severity of HLAP increase with increasing levels of serum TGs; thus, a rapid decrease in serum TG levels is the key to the successful management of HLAP. TPE has emerged as an effective modality for rapidly reducing serum TG levels. However, due to its cost and accessibility, TPE remains poorly evaluated until now [25-27].
Some studies revealed its efficacy in helping to treat and prevent recurrence, while the study of Gubensek et al. suggested that TG levels were not correlated with disease severity, mortality, or length of hospital stay. Garg and Rustagi suggested that TPE should be initiated preferably within 36 h provided that the patient is able to tolerate the treatment. HTG can be effectively controlled through comprehensive treatment, which can not only reduce the risk of cardiovascular events but also reduce microangiopathy related to diabetes and reduce the incidence of diabetes
At approximately the 1-month outpatient follow-up, the patient stated that he was engaging in at least 150 minutes of aerobic activity every week and limited his intake of fat and sugar. His weight was 66 kilograms. Unfortunately, the patient was lost to follow-up in the last half month.
The patient refused genetic testing for blood lipids, blood flow detection, fundus examination and CCTA, so we could not better evaluate the risk of CVD.
Funding information is not applicable.
The authors declare that they do not have any conflicts of interest.
Written informed consent was obtained from the patient.
The work was approved by all the authors.
The work has been approved by all the authors for publication.
Some or all data and material generated or used during the study are available in a repository or online (provide full citations that include URLs or DOI).
Some or all code generated or used during the study are available in a repository or online (provide full citations that include URLs or DOI).
Conceptualization: Mei-lian Cai, Data curation: Mei-lian Cai Investigation: Mei-lian Cai, Resources: Mei-lian Cai Supervision: Min Gong, Writing- original draft: Mei-lian Cai Writing-review & editing: Mei-lian Cai
Citation: Cai ML, Gong M (2022) Hypertriglyceridaemia: A Case Report and Literature Review. J Clin Exp Cardiolog.13.741.
Received: 26-Aug-2022, Manuscript No. JCEC-22-19006; Editor assigned: 05-Sep-2022, Pre QC No. JCEC-22-19006; Reviewed: 19-Sep-2022, QC No. JCEC-22-19006; Revised: 26-Sep-2022, Manuscript No. JCEC-22-19006; Published: 03-Oct-2022 , DOI: 10.35248/2155-9880.22.13.741
Copyright: © 2022 Cai ML, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.