Journal of Infectious Diseases & Preventive Medicine
Open Access
ISSN: 2329-8731
ISSN: 2329-8731
Review Article - (2021)Volume 9, Issue 7
Clinical trials of thermoheliox application (inhalation with a high-temperature mixture of oxygen and helium, 90°C) in the treatment of the acute phase of coronavirus infection were conducted. Dynamics of disease development in infected patients (PCR test for the virus) and, dynamics of changes in blood concentration of C-reactive protein, immunoglobulin M, specific immunoglobulin G were studied. High efficiency of thermoheliox in releasing the organism from the virus and stimulating the immune response (thermo vaccination effect) was shown. The kinetic model of the process is proposed and analyzed.
Coronavirus; Kinetic model; Thermoheliox; C-reactive protein; Immunoglobulin G; pH-jump
Combating coronavirus infection is an extremely acute problem requiring the development of new methods that expand the range of possibilities of modern therapy and prevention [1-10]. Currently used antiviral and restorative therapeutics are often insufficiently effective and lead to deep damage to many body systems and often to the death of the patient [6]. Great hopes are pinned on the development of synthetic vaccines that function at the level of antibody synthesis and specifically interact with certain proteins of the virus.We have developed a new method for combating coronavirus damage to the body, based on the use of thermoheliox (inhalation with a high-temperature mixture of helium and oxygen).The theoretical foundations of the method, experimental observations and results of therapeutic practice are outlined below.
A dynamic picture of the development of acute coronavirus infection
Currently, the etiology of acute coronavirus infection is becoming clearer [8,9]. The disease development process involves several stages:
• The initial stage includes infection and growth of viral concentration. This incubation period can occur without visible clinical changes. Therefore, in the case of a powerful immune system in the body, the virus does not continue to develop. In the second case, the immune system cannot cope with the virus, and viral penetration into the host cells initiates an exponential increase in the concentration of viral particles and the transition from the mild to the severe form of the disease.
• The next stage is destruction of the host cells by the virus and accumulation of dead cells.
• Under intensive aerobic conditions, necrotic biochemical processes of oxidation take place in dead cells, including lipid peroxidation, release of organic acids of the tricarboxylic acid cycle, ATP (adenosine triphosphate) hydrolysis with formation of phosphoric acid and other acidogenic processes.
• Local acidification of the lesion zone occurs.
• All enzymes with the imidazole group of histidine (pKa˜7.0) in the active center, particularly the key enzymes of carbonic anhydrase and plasmin in the plasminogenic fibrinolytic complex, stop working in the lesion zone [11,12]. The protonation of these enzymes blocks their catalytic activity, increasing the concentration of hydrogen ions. The lesion zone stops releasing CO2 into the gas phase and accumulates bicarbonate.
• Microvessels are clogged in the lesion zone. Thrombus formation means that the plasmin-plasminogenic system that continuously dissolves fibrin clots and blood clots in the body is locally “turned off”. Disabling the plasminplasminogenic system has the same effect as the “shutdown” of carbonic anhydrase. The active center of the plasmin contains an imidazole group of histidine, the protonation of which reversibly blocks the activity of plasmin in the lesion zone. Plasmine stops hydrolyzing fibrin clots, and thus a blood clot occurs.
• Hemoglobin is a pH-sensitive component of the system. With an increase in the concentration of hydrogen ions (decrease in pH), the ability of hemoglobin to bind oxygen decreases (Bohr effect, 1904). So when the pH changes by 0.2 units. (from pH 7.4 to pH 7.2) the ability of hemoglobin to bind an oxygen molecule decreases by 2 times.
We have developed a kinetic model of the development of acute coronavirus infection [13-15]. The model includes differential equations for the growth of the infectious virus concentration, the lung cells metabolically destroyed by the virus, the growth of pathogenic microorganisms that use the affected cells as a “substrate” for growth [16-18]. The principal participant in the process is the carbonic anhydrase enzyme, which carries out the process of bicarbonate ion conversion into carbon dioxide gas withgeneration of hydroxyl ions. Blocking of this enzyme by reducing the pH (acidification of the lesion zone) results in complete respiratory arrest [19,20].
The key process in the development of pathology is the dynamics of pH changes in the lesion zone. The rate of change in the concentration of hydrogen ions depends on the buffer properties of the blood system and the dynamics of proton emission by dead (necrotic) cells. The reaction of carbonic anhydrase, which produces hydroxyl ions, counteracts the process of “acidification” of the lesion zone of the lungs. The activity of this enzyme depends on pH and at pH below 7.0 the enzyme practically stops working.
The solution of the system of equations allows obtaining a kinetic description of the observed phenomenon of pathology development due to infection of the organism by coronavirus. One of the process scenarios is presented in (Figure 1) [13-15].
The main result of kinetic analysis and mathematical experiments is the demonstration of a jump-like change in pH upon reaching a certain level of damage (curve H+, Figure 1). This is the death of the body with the shutdown of all enzymes and proteins that have an imidazole group of histidine in the active center (carboanhydrase, hemoglobin, plasmin, etc.). This means the termination of the binding and transfer of oxygen by hemoglobin, the activation of thrombus formation processes due to the loss of the activity of the thrombolytic enzyme plasmin. This process occurs a jump-like manner.
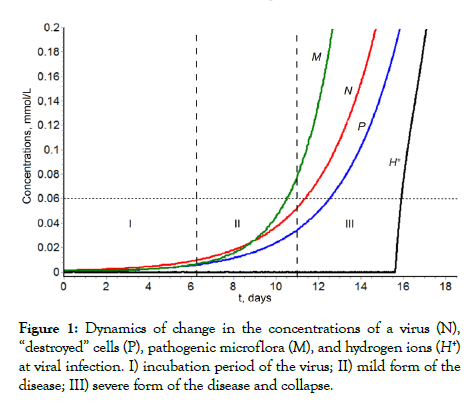
Figure 1: Dynamics of change in the concentrations of a virus (N), “destroyed” cells (P), pathogenic microflora (M), and hydrogen ions (H+) at viral infection. I) incubation period of the virus; II) mild form of the disease; III) severe form of the disease and collapse.
The obtained solution qualitatively describes the phenomenon of infection and disease development:
1. Incubation period during which practically no signs of the disease are observed. From (dashed line) it is observed that with the parameters in [14] this period lasts 150 hours (approximately 6.5 days) (Figure 1).
2. At the end of the incubation period (induction period), rapid symbiotrophic growth of the virus concentration and pathogenic microflora concentration with significant accumulation of virus and microorganisms is observed.
3. In primary periods of pathology development (the incubation period and initial period of active growth of the virus and pathogenic microflora concentrations), the pH value in the lesion zone is in the range 7.4-7.2. In the absence of treatment and a purposeful effect on the system behavior, bifurcation growth and pH blocking of carbonic anhydrase activity should be observed. As a consequence, complete respiratory failure occurs. With the given parameters [14], the model predicts that respiratory arrest should be observed on the 13th-16th day after infection. The collapse has a bifurcated character, and the transition point is clearly identified graphically (Figure 1). The bifurcation point is a fundamentally important characteristic of the process and is the point of “no return”, i.e., the time of death of the body. When passing through this point, the release of CO2 from the liquid phase to the gas phase is stopped. Uncontrolled growth occurs in the bicarbonate concentration in the blood, i.e., complete blocking of breathing occurs.
The nature of the therapeutic effect when the body temperature rises
The natural process of disease development is associated with an increase in body temperature. The inflammatory response is initiated by a large complex of biochemical reactions, including the synthesis of inflammation mediators of the prostaglandin type [21,22], synthesis of heat shock proteins, and activation of the immune system.
The increase in temperature affects the increase in the rate of thermal death of microorganisms and viruses. It is known that as the temperature increases the concentration of microorganisms and viruses decreases exponentially as a result of thermal death.
Microorganisms, including pathogens, are highly sensitive to temperature increases. Thus, the rate of death of Escherichia coli at a transition from 54°C-60°C increases by 14.3 times. The rate of death of Staphylococcus aureus increases by almost 5 times with an increase in temperature from 53°C -57°C. From the data presented in [23], we can estimate that ΔH*M = 100kcal/mol (thermal death of S. aureus) and ΔH* M =118 kcal/mol (thermal death of E. coli). If we assume that the thermal death of viruses is determined by the thermal degradation of capsule proteins, it can be accepted that ΔH* N =40 −50 kcal/mol.
Within the discussed model, it appears possible to consider the effects of direct inactivation of viruses and pathogenic microorganisms at the transition from the “normal” temperature of 36°C to the temperature of the inflammatory process, 41°С. It is well known that an increase in temperature to 40°C-42°C is an important factor in the development of inflammatory process [22]. If the activation energy of thermal destruction of viruses is conditionally taken to be equal to 40 kcal/mol, then the transition from normal temperature to inflammation temperature increases the rate of virus destruction by 2.8 times. Calculations show that in this case, the bifurcation point of collapse shifts from 360 hours to 420 hours. At an activation energy of 50 kcal/mol, the rate of virus destruction increases by 3.5 times and the collapse shifts to 440 hours. Thus, the patient gets significant additional time to Figureht the disease. In any case, an increase in temperature leads to thermoinactivation of the virus and an increasing the patient's life time.
Within the kinetic model, it is possible to estimate the time period of temperature increase (Δtther), necessary for the therapeutic effect Δtther
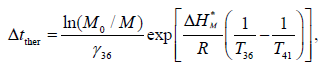
where M0/M is the depth of lung cleanse from the microbiological pathogen, γ36 is the kinetic parameter of thermal death (5.10–3 h–1), ΔH* M is the activation energy of the thermal death of the microorganism, and T36 and T41 are the patient's temperature in normal conditions and with the increase in temperature, respectively. Estimates show that the depth of inactivation of the microorganism at 10 times (M0/M)=10 will be achieved in 34 hours; at 100 times, in 65 hours.
Therapeutic effect of thermoheliox
The use of thermoheliox, which is a thermolized mixture of helium and oxygen, appears to be the most promising therapeutic means of suppressing viral growth. The essence of the approach is to influence the patient's respiratory system with a thermoheliox at relatively high temperatures. The therapeutic procedure consists of supplementing the patient's breathing with a helium-oxygen mixture (80%-60% helium, 20%-40% oxygen) at gas-mixture temperatures of 50°C-90°C.
The methodology of using a thermolized mixture of helium and oxygen has a detailed scientific justification and has found highly effective application in the treatment of pathologies of the respiratory system, ischemic stroke [23-28]. Thermoheliox is also used for treatment of pregnant pathologies (A.A. Panin et al, unpublished data). The successful therapeutic application of the method was reported for more than 2500 patients in the pulmonology and neurology departments of D.D. Pletnev City Clinical Hospital (Moscow) [24-28]. A unique medical device that allows regulation of the composition and temperature of the helium-oxygen mixture and drug delivery to the lungs was developed [29]. The available experimental experience and practice (use in saunas for breathing air at up to 100°C) demonstrate that a thermolized mixture of oxygen and nitrogen is practically harmless to the human body within 20-30 minute of exposure. Safety of using of breathing air heated to high temperatures is confirmed by centuries of experience with saunas. This is also confirmed by clinical experience [24-28].
A thermolized mixture of helium and oxygen behaves in a similar way. Helium, with its high diffusion capacity, drains well, bypasses all body tissues, and significantly improves microcirculation in all organs and tissues. Thermoheliox significantly improves oxygen delivery, reduces airway resistance, improves the ventilationperfusion ratio through the alveolar-capillary membrane of the lungs and normalizes the acid-alkaline state. Thermoheliox is much more effective than a mixture of oxygen and helium at room temperature. Mammalian and human cells use specialized protection mechanisms against temporary overheating (heatshock proteins). At the same time, the virus is effectively destroyed by denaturation of proteins and nucleic acids. For example, the flu virus at 50°C-60°C “lives” for a few minutes, the HIV virus is inactivated by a factor of 100 at 56°C for 30 minutes, the hepatitis virus loses its activity at 100°C for 2 minutes, and foot-and-mouth disease virus is destroyed at 50°C-60°C in 5-10 minutes [19,23].
Thermoheliox does not cause destruction of cells of the human respiratory system thermoheliox proteome
It was made a pilot study to assess the effect of thermoheliox on the state of the respiratory tract by studying of the exhaled breath condensate protein composition before the thermoheliox procedure, immediately after and after three hours of relaxation.
A comparative study of the Exhaled Breath Condensates (EBC) protein composition of non-smoking healthy donors was carried out. The EBC was taken before the respiratory procedure, immediately after a 20-minute inhalation by mixture of He/O2 gases (70/30) heated to 70°C and 3 hours later. The protein composition was determined by chromatographymass spectrometric analysis after selective tryptic hydrolysis. The results were processed using the Mascot program and the UniProt database [29-40].
After the heliox procedure, the volume of the collected condensate (1-1.5 ml) decreases by an average of 30% and is practically restored after three hours of relaxation. Most proteins were consistent for all samples, regardless of the thermoheliox procedure. These are keratins, several proteins of the immune system (immunoglobulins, compliment proteins), tubulin. In samples after thermal heliox, the appearance of small amounts of additional proteins is observed. These are proteins of muscle metabolism (actin and calmodulin), fibrinogen, traces of hemoglobin, apolipoprotein, type B creatine kinase. After three hours of relaxation, tubulin disappears in the EBC [41,42].
Most exhaled proteins are the same before, after the procedure, and for three hours of relaxation. The results obtained demonstrate the relative safety of the use of high-temperature heliox as a therapeutic agent [41,42].
Thermoheliox destroys the virus in the body
Clinical trials were performed on the basis of the intensive care unit of the N.V. Sklifosovsky Clinical and Research Institute for Emergency Medicine, Moscow (prof. S.V. Zhuravel). It has been shown that inhalation with high-temperature thermoheliox (90°С) significantly reduces the concentration of coronavirus in the body.
The study included 60 patients with COVID-19, who were divided into two equal groups. The first (“working”) group (N=30) included the patients who received thermoheliox therapy in the standard COVID-19 treatment protocol, and the second (control) group (N=30) included the patients who received the standard therapy according to the temporary methodological recommendations of the Ministry of Health of the Russian Federation.
Of the 60 patients included in the study, 28 (46.7%) were medical professionals. The male/female ratio was 17/13 in the “working” group and 16/14 in the control group. In both groups, patients were sexmatched(p=0.403 according to Fisher’s exact test). The mean age of patients in the study was 56.7 years (45 years; 61 years). The mean age of patients in the “working” group was 58 years (45 years; 59.5 years); in the control group, 55 years (46 years; 66 years). The patients were comparable in age. The severity of lung damage at the time of inclusion in the study was 25.2% (21%, 42.5%) in the “working” group and 26% (25%, 41.7%) in the control group. General clinical symptoms of patients in both groups included the feeling of loss of smell and taste, runny nose, feeling short of breath, shortness of breath, weakness, fever, headache, pain in muscles and throat, and dry cough.
All patients included in the study protocol no. 11-20 dated April 20, 2020 were treated for pneumonia caused by the SARS–CoV 2 virus from April 21 to June 2020 inclusive.
All patients underwent swabbing from the nasal cavity and oropharynx mucosa to detect SARSCoV-2 coronavirus RNA by PCR in the CFX-96 real-time PCR detection system (Bio-Rad, United States)) [43-45]; computed tomography (CT) of the lungs [46]; and venous blood sampling for analyzing the content of immunoglobulins IgG (sc) and IgM (sc) to the SARS-CoV-2 spike S protein using the standard enzyme-linked immunosorbent assay (ELISA) with a Mindray 6000 immunochemiluminescence analyzer (United States) [45]. ELISA testing for the presence of IgG helps to detect the contact of the body’s immune system with the virus if 2 weeks have passed since the time of infection.
Data were statistically processed using the SPSS 17.0 software package (SPSS Inc., United States). Methods of nonparametric statistics included the Mann–Whitney U test (comparison of two independent variables) and Fisher’s exact test. Differences were considered significant at p<0.05.
The dynamics of changes in the number of patients in the control and “working” groups with a positive test for SARSCoV-2 RNA (Figure 2). In the “working” group, a significant decrease in the number of patients with a positive test for SARSCoV-2 RNA was observed.
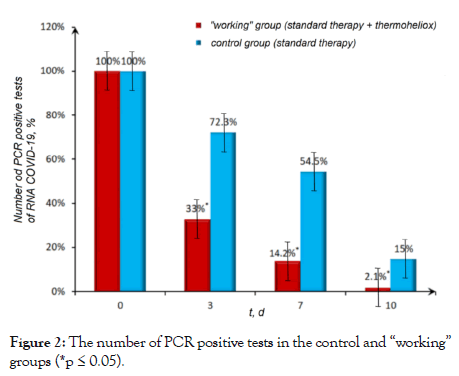
Figure 2: The number of PCR positive tests in the control and “working” groups (*p ≤ 0.05).
According to our observations, in the “working” group of COVID-19 patients who received inhalations with thermoheliox, a negative PCR result was obtained on the third day, and in some patients a negative result was detected as early as on the first day after the start of therapy. Against the background of the standard therapy in the control group, a positive reaction to corona viral RNA was detected from 7 days to 4 weeks after the onset of the disease, in some cases even longer [47].
Thermoheliox as an anti-inflammatory agent. stabilization of C-reactive protein level
The immune response of the body is complex and involves switching on various biochemical systems of the body [48,49]. In particular, the C-reactive Protein (CRP) is considered as one of the components of the complex chain of biochemical processes, one of the first to respond to bacterial and viral infections. CRP synthesis is induced by cytokines and by lung tissue destruction [50,51]. We investigated the comparative dynamics of the accumulation and reduction of CRP in the course of the standard treatment and the treatment with the thermoheliox inhalations. A fundamental difference in the response dynamics was observed (Figure 3).
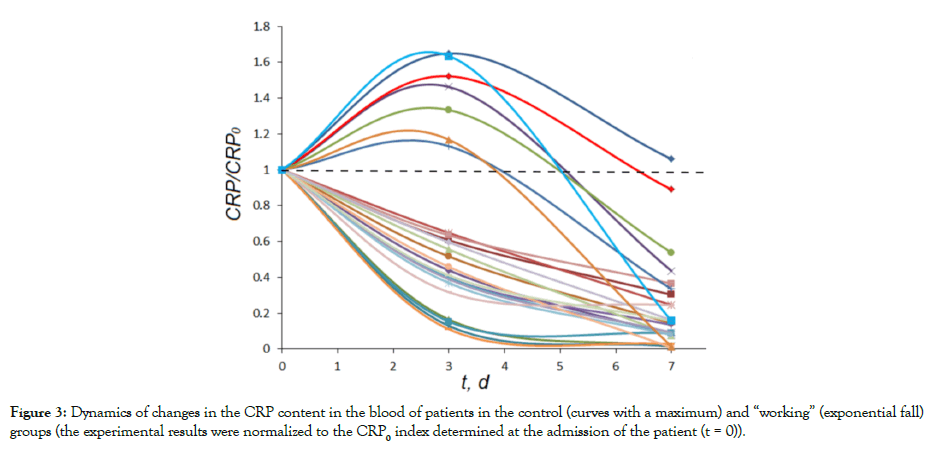
Figure 3: Dynamics of changes in the CRP content in the blood of patients in the control (curves with a maximum) and “working” (exponential fall) groups (the experimental results were normalized to the CRP0 index determined at the admission of the patient (t = 0)).
It can be seen that, during the “control” course of the disease in the control group, in most cases, CRP accumulation was relatively slow and reached a maximum on days 2-4 of treatment (Figure 3, curves above the dotted line). The subsequent process of treatment in dynamics is characterized by a decrease in CRP to zero. Thermoheliox inhalations in the “working” group stimulated transition of the system from the very initial period to an exponential decrease in the CRP level (Figure 3, curves below the dotted line).
Thermoheliox stimulates the formation of antibodies (IgM and specific antibodies IgG). thermovaccination
The results obtained demonstrate that the use of thermoheliox effectively stimulates the synthesis of antibodies of both types (immunoglobulin G and immunoglobulin M) [47-53]. Some of the patients were admitted to the hospital with already formed high levels of IgG and IgM. Both in the “working” and in the control group, the proportion of such patients was approximately 25%. For kinetic analysis, data with a complete set of antibody level measurements (four measurements: at admission, on days 3 and 7, and at discharge) were suitable. The experimental data on the dynamics of IgG accumulation in the control (Figure 4A) and “working” (Figure 4B) groups. A fundamental difference in the kinetics of the immune response can be seen.
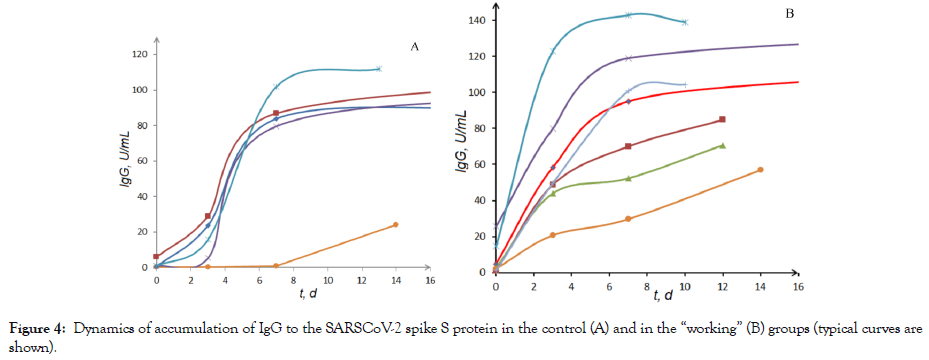
Figure 4: Dynamics of accumulation of IgG to the SARSCoV-2 spike S protein in the control (A) and in the “working” (B) groups (typical curves are shown).
When the patients of the control group were treated without inhalations with thermoheliox, the synthesis of IgG in the first three days was practically blocked (the induction period on the kinetic curve of IgG accumulation). The use of thermoheliox (four 15-min inhalation procedures with a 15-min break) by the patients of the “working” group eliminated the induction period and triggered the synthesis of immunoglobulin G starting from the first procedure. It should be noted that approximately 30% of patients are admitted to the hospital with already completed stage of the immune response activation (the induction period on the kinetic curves of IgG accumulation is not observed) [48].
Thermoheliox also stimulates the production of IgM. The differences in the kinetics of IgM accumulation in the “working” and control groups are not as significant as in the case of IgG. Thermoheliox markedly stimulates the accumulation of IgM at the initial stage of the development of the process. For example, the mean IgM(3)/IgM(0) value for the entire population of data for the patients who underwent inhalations with thermoheliox was 4.1 (in the control group, 3.2). The results on the dynamics of the synthesis of IgG and IgM clearly demonstrate that the use of thermoheliox in the treatment of coronavirus infection leads to activation of the immune system and stimulates the production of specific antibodies.
Stimulation of the immune response by thermoheliox can be defined by the term thermo vaccination. We have shown experimentally that, on days 2-3 of using thermoheliox, in most cases, the body is freed from viral particles, judging by the results of PCR analysis. We assume that the accelerated production of antibodies takes place, apparently, against the proteins that are the products of thermal destruction of viral particles in the lungs of patients. Thus, in the case we are discussing, we deal with the “classical” vaccination with a weakened or destroyed antigen. A fundamentally positive difference lies in the fact that the process takes place in vivo with the involvement of natural viral proteins, and thermovaccination can have a wide range of specificity.
The mechanism of the observed effect of the stimulation of immune response by thermoheliox requires further investigation. The kinetic model developed and investigated by us [13-15,52] explains the observed effects by an increase in the antigen concentration during the thermal destruction of the virus.
The effects of thermo vaccination in the treatment of coronavirus lesions (stimulation of the immune response by thermoheliox), which were discovered and described by us for the first time, may have a general nature and can be used in the treatment of lesions by viruses of another nature.
Potential development
The currently developed procedure of using high-temperature thermoheliox finds a fairly reasonable and widespread application for the treatment of diseases of the respiratory system [28,30,31,52], ischemic strokes [25,28]. We demonstrated that high-temperature thermoheliox is an effective means of combating coronavirus infection [13-15, 53]. It can be predicted with a reasonable degree of confidence that this methodology, which provides an improvement in heated oxygen access to various organs of the body and has a therapeutic effect, can be used in various fields of medicine, including
• Viral diseases of various nature;
• Various pulmonary infections;
• Tuberculosis;
• Neurodegenerative diseases.
At present the methodological and theoretical foundations for the wide use of this methodology have been created.
Stimulation of the immune response by thermoheliox can be defined by the term thermo vaccination. We have shown experimentally that, on days 2-3 of using thermoheliox, in most cases, the body is freed from viral particles, judging by the results of PCR analysis.The effects of thermo vaccination in the treatment of coronavirus lesions (stimulation of the immune response by thermoheliox), which were discovered and described by us for the first time, may have a general nature and can be used in the treatment of lesions by viruses of another nature.
Citation: Varfolomeev SD, Tsybenova SB (2021) High-Temperature Thermoheliox in the Treatment of Coronavirus Infection: Thermovaccination- Thermoheliox as a Stimulator of the Immune Response. J Infect Dis Preve Med. 9:229.
Received: 01-Jun-2021 Accepted: 15-Jul-2021 Published: 22-Jul-2021 , DOI: 10.35248/2329-8731.21.9.229
Copyright: © 2021 Varfolomeev SD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.