Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Research Article - (2020)Volume 10, Issue 4
Background: Henoch-Schönlein Purpura (HSP) is the most common childhood vasculitis. It is a systemic vasculitis with multiorgan involvement. Diagnosis is based on clinical criteria. The classic tetrad of signs and symptoms includes palpable purpura, arthritis or arthralgia, abdominal pain and renal disease. Other organs involvement such as brain, lungs, and scrotum are less common but may be occasionally involved.
Methods: Retrospective study of 100 patients that were hospitialized and diagnosed with HSP between 2006-2018 in Poria hospital included in the research. Patients were chosen based on EULAR criteria.
Results: 100 patients entered the study, with male predominance (M:F ratio of 1.17). The average age was 6.3 and the median age 6. 88% of patients were under the age of 10 when diagnosed. Average length of stay in the hospital was 2 days. Most of the patients presented in the winter and spring month (October-March). The most frequent laboratory abnormality was leucocytosis (30%), and 2% of patients had proteinuria, but none had renal injury. Clinically, all of the patients had palpable purpua (a mandatory criteria according to the EULAR), 67% had joint involvement, 32% had abdominal pain and 11% of boys had testicular pain. A statistically significant correlation was demonstrated between joint pain and increase signs of inflammation, between anemia and fever and between abdominal pain and chances of future relapses. Not a single patient developed acute or chronic renal failure.
Conclusions: The epidemiological, laboratory and clinical presentation in this study were not different from what described in the literature and other similar studies around the globe. We did found a statistically significant correlation between presentation of abdominal pain to relapses of the disease in the future. Not a single patient developed acute or chronic renal failure.
Henoch-shonlein purpura disease; Literature review; Vasculitis; Purpura; Immunoglobulin A
Henoch-Schönlein purpura (HSP) is an acute, systemic, immune complex-mediated, leukocytoclastic vasculitis [1,2], characterized by the deposition of immunoglobulin A (IgA)-containing immune complexes in the walls of small vessels [3]. Although it occurs mainly in children, it is also seen in infants, adolescents, and adults. Several authors have used the term “Acute Hemorrhagic Edema of Infancy” (AHEI) if the children were less than 2 years old. The age of onset has been considered to be an important factor in disease severity and outcome.
The majority of HSP cases are preceded by an upper respiratory tract infection suggesting a potential infectious trigger. Streptococcus, staphylococcus and parainfluenza are most commonly implicated [1-3].
The characteristic pathological feature of HSP vasculitis is a deposition of IgA-containing immune complexes in vessel walls of affected organs and in the kidney mesangium. The genetic background of the disease include several polymorphisms with influence on the disease susceptibility, severity and risk of renal involvement. The increased risk of developing HSP were associated with polymorphisms in the HLA groups (mainly B35 and DRB1), but studies from Israel and Turkey showed also linkage to mutations in the MEFV gene [4-8]. Abnormal IgA1 glycosylation is believed to be a leading phenomenon in the pathophysiology. The classic tetrad of signs and symptoms includes palpable purpura, arthritis or arthralgia, abdominal pain and renal disease [3]. Other organs involvement such as brain, lungs, and scrotum are less common but may be occasionally involved [1,3,4]. A non-migratory arthritis occurs in 75 percent of patients with HSP. The knees and ankles are more commonly involved than small joints. The arthritis symptoms include swelling, warmth, and tenderness. The symptoms are transient, leave no deformity, and may precede the purpuric rash in 15 to 25 percent of patients [1-3]. Approximately 60%-65% of HSP patients develop abdominal pain. One explanation to these symptoms is immune complex deposition in the intestinal vessel walls. The pain is usually diffuse, increasing after meals.
Renal disease is the most serious sequela of HSP occurring in 20 to 55 percent of patients. The most common finding is isolated microscopic hematuria, but mild proteinuria, nephritic/nephrotic syndromes and hypertension were also reported. Most HSP nephritis (HSPN) is mild, and usually remits within 6 months, although progressive glomerulonephritis leading to renal failure have been described [1-3]. The risk factors were varied, and include epidemiological risk factors (older age, especially age above 10, male gender and relapse) clinical manifestations (persistent purpua and GIT symptoms-abdominal pain, GIT bleeding and severe bowel angina) and laboratory abnormalities (WBC>15 K, platelets>500 K, positive ASLO test and decreased C3) [9-11].
Though less common, one organ involvement that is worth mention (one that was also examined in our research) testicular involvement. The manifestations are testicular pain, swelling and orchitis. Until 2008, diagnosis was based on the criteria proposed by a committee of the American College of Rheumatology (ACR) held in 1990. Those criteria was, however, not specific enough, and over the years different researchers and conferences tried to address this problem, but none has proven to be good enough [12,13]. In 2008, a summit held at Ankara proposed revised criteria-the EULAR/PRINTO/PRES criteria of HSP. Those criteria were validated in 2010, and they are the gold standard for HSP diagnosis [14,15]. The sensitivity is 100% and specificity 87%, when applied to children [14]. Regarding treatment, several postulation can be made.
The general agreement is to give supportive and symptomatic treatment as long as renal involvement is absent. Example for Such therapy is pain medication, rehydration (per OS or intravenous) and surgery in cases of complication such as testicular torsion or intussuption [1-3]. Steroids use has been source of controversy for many years. As for renal involvement, steroid use demonstrated no long-term benefit over placebo in terms of renal manifestations, but did showed faster resolution [16]. Other immunosuppressive and immune-modulatory drugs were tried in HSP with renal involvement, and general statements regarding them can be made. Cyclosporine A have proved efficacy in HSPN in terms of proteinuria resolution. In contrasting cases of HSPN with persistent proteinuria, Angiotensin-Converting Enzyme Inhibitors (ACEI) or (ARB’s) has become an accepted treatment, with the possibility of renal fibrosis inhibition [3]. In extra-renal Angiotensin Receptor Blockers involvement (abdominal and joint pain, but not skin manifestations), studies of recent years show significant improvement in patients receiving corticosteroid vs. placebo [1,17,18]. As for skin manifestations, case reports support the use of Dapsone (drug known for its anti-inflammatory and immunomodulatory properties), with good outcome. Other anecdotal reports support the use of Rituximab, an anti-CD20 antibody which function as B-cell inhibitor [19].
Statistical methods
Quantitative (continuous) variables were expressed as mean, median, Standard Deviation (SD) and average. Qualitative (categorical) variables were expressed as percentages and frequency. Data were analysed using Comparative methods.
A total of 100 patients with HSP diagnosis by EULAR criteria included in the study. The main epidemiological information is shown in Table 1. Between January 2006 to December 2018 100 children fulfilling the inclusion criteria were hospitalized and treated in Poria hospital. There was slightly male predominance (M:F ratio of 1:17) the age range was 1-17 (mean age 6.3 and median age 6).
| Age ( years) | |
| Mean ± SD | 6.32 ± 3.1 |
| Range | 1-17 |
| Median | 6 |
| Sex | |
| Male/female | 54/46 |
| Proportion of boys | 54% |
| Ratio M:F | 1.17 |
| Hospital duration | |
| Mean ± SD | 2.22 ± 1.31 |
| Range | 1-7 |
| Median | 2 |
| Seasonal pattern | |
| Summer | 21 |
| Autumn | 33 |
| Winter | 27 |
| Spring | 19 |
| Ethnicity distribution | |
| Arabs | 54 |
| Jews | 46 |
| Habitat | |
| Rural | 54 |
| Urban | 46 |
| Recurrence rate | 9 |
Table 1: Epidemiological data in 100 children with HSP.
Most of the cases occurred in the winter and autumn correlating with known literature. Most of the patients were under the age of 10 (88%). Arab patients were slightly more affected than jewish patients (54% vs. 46%, respectively). Median length of stay in the hospital was 2 days, and most of the patients were hospitalized up to 3 days (85%). As for place of living, overall there was a tendency
Lastly for epidemiological data, 9 patients had recurrence and a strong correlation was found between abdominal pain and recurrence (statistically significant, explained later).
Laboratory findings
Main laboratory findings are shown in the Table 2. 30% of patietns had leukocyotiss, anemia was present in 19%, and signs of inflammation, measured as either increase in C-Reactive Protein (CRP) or increase in Erythrocyte Sedimentation Rate (ESR) was 27%. Proteinuria was noticed in 2 patients, neither of them had renal insufficiency and evidence of recent streptococcal infection was identified in 22%.
| Findings | No. positive/No. tested (%) |
|---|---|
| leukocytosis | 30/100 (30%) |
| Anemia elevated | 19/100 (19%) |
| ESR/CRP | 27/100 (27%) |
| positive ASLO test | 16/100 (16%) |
| positive throat culture | 6/100 (6%) |
| proteinuria | 2/100 (2%) |
Increase in WBC (leukocytosis) was defined as WBC>10 × 103/mm3 Signs of inflammation were defined as elevated ESR or CRP: Elevated CRP=CRP>5 mg/L Elevated ESR=ESR>20 mm/H Positive ASLO test were defined as=ASLO>200 U/ml Proteinuria-protein>300 mg/D in urinalysis
Table 2: Laboratory findings in 100 children with HSP.
Clinical features
By using the EULAR criteria, all patients had palpable purpura (100%), so a graph showing percentage of pupura was not needed. 67% of patients presented with joint manifestations, either arthritis or arthralgia (no distinct separation was made). Abdominal pain was noticed in 32%, none of which needed surgical intervention or was diagnosed with intussusception. Data regarding GIT bleeding was missing. 11% of male patients complained of testicular pain, which correlate with the literature. None of the patients had orchitis or testicular torsion, so no surgical intervention took place. Fever (temperature above 38 celcius) is measured in 14% of patients (Figure 1).
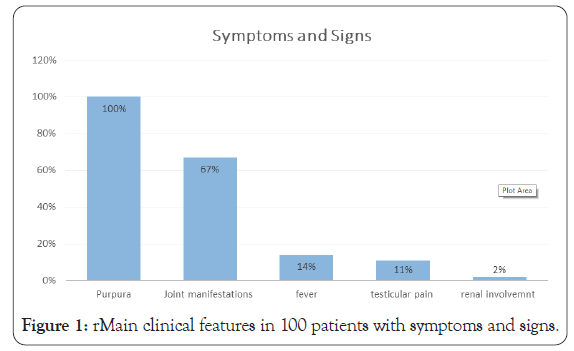
Figure 1: rMain clinical features in 100 patients with symptoms and signs.
Statistical significance was defined as P-value <0.05
• Hemoglobin vs. fever-the study shows a strong correlation between presenting with anemia and fever. If the patient has anemia, he has more chances of having fever on presentation (42%) than the opposite (31%), but both shown enough dependence (Figure 2).
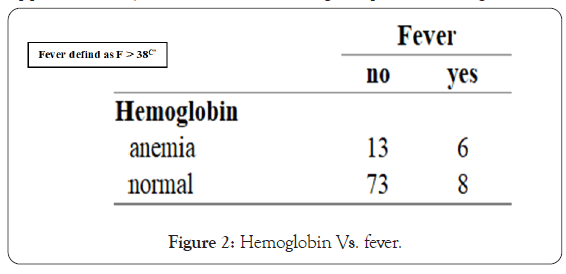
Figure 2: Hemoglobin Vs. fever.
• CRP vs. joint manifestations-the study shows a strong correlation between joint manifestations and increase in CRP. If patient presented with joint pain, he had more chances of having his CRP increased in blood test (865), which correlate pretty well with the logical idea of that person suffer from joint inflammation (arthritis) (Figure 3).
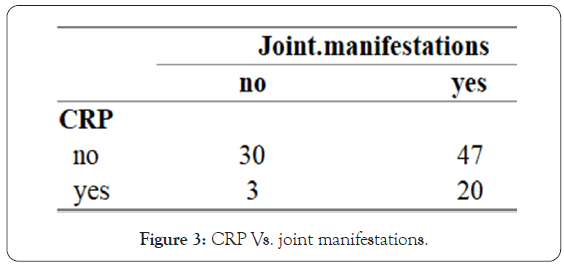
Figure 3: CRP Vs. joint manifestations.
Abdominal pain vs. recurrences
• Perhaps the most important outcome of the research shows a strong correlation between presentation of abdominal pain and the chances of HSP recurrences in the future. If the patient presented with abdominal pain, he had greater chances of having recurrences (88%) than if not. In the studies reviewed while doing this research, this information was not mentioned (Figure 4).
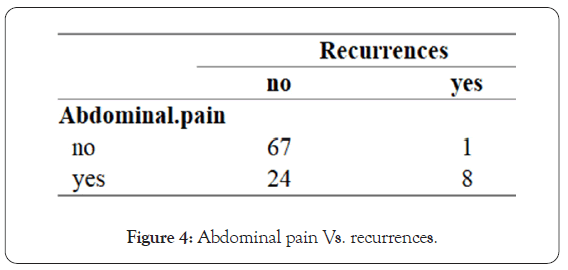
Figure 4: Abdominal pain Vs. recurrences.
In our study, 100 patients were diagnosed with HSP in our hospital over the years 2006-2018. Most of our data fitted the known literature. 88% of the children were under the age of 10 (90% in most studies), the disease was mild in all of them. The disease picked in the winter (late autumn) and spring, and many patients demonstrated signs of upper respiratory tract infection (22%). Clinically, all of them had palpable purpua, 68% presented with joint involvement (70%-75% in the literature), but only 35% presented with abdominal pain (which is in contrary to studies and literature. also, only 2% had renal involvement, and none had chronic renal damage. 11% had testicular involvement.
From laboratory point of view, 30% of patients had leukocytosis, 19% had anemia and 28% had signs of inflammation. Proteinuria was noticed in 2% of patients.
Complex analysis showed statistical significance link between anemia and fever and between CRP and joint involvement, but most importantly, between abdominal pain and the chances of relapses. This data did not appear in the literature review the author did.
Citation: Sleman B, Berkovich A, Nasser S, Boshra N, Haia N, Nasser W (2020) Henoch Schonlein Purpura in Children Ages 2-18 in Poria Hospital: Clinical Manifestations of 100 Cases Over a 12 year Period. Rheumatology (Sunnyvale). 10:269.
Received: 26-Oct-2020 Accepted: 09-Nov-2020 Published: 16-Nov-2020 , DOI: 10.35248/2161-1149.20.10.269
Copyright: © 2020 Sleman B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.