Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Research Article - (2019)Volume 9, Issue 1
Introduction: 387 million people are considered to have diabetes all over the world, and the number is expected to increase miserably to 592 million by 2035. Alternative ways to create β-cells from endogenous sources should be found as a way for the evolution of treatment. This is to bypass the complication of tissue matching and surgical procedures. To date several rebuilding approaches have been developed to stimulate β-cells regeneration through the induction of the proliferation of remaining β-cells, neo-genesis; de novo islet formation from pancreatic progenitor cells, and trans-differentiation; converting non-β-cells within the pancreas to β-cells. Platelet Rich Plasma (PRP) contains various growth factors which can be used for tissue regeneration including pancreatic beta cells.
Materials and methods: 2 groups of Type-2 diabetes patients had been monitored in a private clinic, number 40 each group, with 30 females and 50 males. The first group patients relieved oral hypoglycemic drugs as usual but injected by PRP weekly by subcutaneous injection of 3 ml, the second group received oral drugs only.
Results: There were significant increases in c-peptide levels in patients with PRP injection with dpp4 inhibitors and metformin for 3 months with p-value less than 0.0001. In the second group patients on oral therapy only there were no significant change in c-peptide levels after 3 months of oral hypoglycemic drugs.
Conclusion: As Growth factors (GFs) are considered as a natural biological mediators which control growth, differentiation, and have a role in the process of tissue reform and regeneration. The growth factors in platelet-rich plasma can induce beta cell regeneration and increase beta cell mass by stimulating β-cell neo-genesis and through ductal cell differentiation into β-cells which is detected by an increase in c-peptide levels which may add to Type-2 diabetes treatment.
Platelet Rich Plasma (PRP); Type-2 diabetes; β-Cell mass; Growth factors (GFs); c-Peptide
Around the world, 387 million people are considered to have diabetes, and the number is expected to increase miserably to 592 million by 2035 [1]. We can say actually that Type-2 diabetes represents about 90% of cases of diabetes [2].
Type-2 diabetes is deemed really to be a critical chronic disease due to inheritance interaction and complex environment along with other risk factors such as obesity and a sedentary lifestyle. Type-2 diabetes is considered as a major health problem, with a Type-2 diabetes is deemed really to be a critical chronic disease due to inheritance interaction and complex environment along with other risk factors such as obesity and a sedentary lifestyle. Type-2 diabetes is considered as a major health problem, with a
The impaired insulin secretion in Type-2 diabetes (T2D) is presumably occurs as a result of a decrease in the secretory rate cellular of beta cells (that is, in individual β-cell function), or to a decrease in β-cell mass (the product of β-cell size and number), or both. Whereas much debate has been sitting about the relative contributions of secretory dysfunction and loss of β-cell mass to impaired insulin secretion in T2D, a consensus view is still lacking. This may be due to the hardness in getting human islets of convenient quality and quantity for studies because islet isolation programs primarily work to get islets from suitable granters for transplantation [4].
It is considered that the decrease in β-cell volume density (β-cell decline) by 30% detected in patients with Type-2 diabetes contributes to the impaired insulin secretion [5]. Alternative ways to create β-cells from endogenous sources should be found as a way for the evolution of treatment. This is to bypass the complication of tissue matching and surgical procedures [6].
There is constant attention in perception of the procedure of endogenous extension of cells to rejuvenate their endogenous mass. Beta cell mass is specified as the total weight of cells within a pancreas and is specified by the equilibrium between death apoptosis and birth proliferation of situated cells [7].
Stimulating β-cell reproduction is an outright and efficient way to raise absolute cell mass in mouses however in human islets it has been totally complex [8].
A different plan is to regenerate the cell mass by differentiation of new cells. It is settled that there is not an adequate evidence that adult pancreatic stem cell can bring about ductal endocrine and exocrine lineages. While enormous work favors new cell formation from progenitors within the pancreas or by transdifferentiation of differentiated pancreatic cells into functional cells [9].
To date several rebuilding approaches have been developed to stimulate β-cells regeneration through the induction of the proliferation of remaining β-cells, neo-genesis; de novo islet formation from pancreatic progenitor cells, and transdifferentiation; converting non-β-cells within the pancreas to β- cells. A pattern of stimulation is common, direct, and least invasive ways to increase β-cell mass [6].
Growth Factors (GFs) are considered as a natural biological mediators which control growth, differentiation, and have a role in the process of tissue reform and regeneration [10].
Growth factors could influence the growth of cells. This definition has extended to include secreted molecules that boost or repress mitosis or impact cellular differentiation. Growth factors work on certain receptors that transmit their growth signals to other intracellular components and ultimately result in the general process of transmitting an external molecular signal to a cell to trigger a cellular reaction called Signal Transduction [11].
Long-term use of a low dose of Epidermal Growth Factor (EGF) stimulates β-cell neo-genesis in diabetic mice and induces ductal cell differentiation into β-cells [12]. It is detected that Hepatocyte Growth Factor increases cell proliferation in adult transgenic mice in basal conditions [13]. Platelet Rich Plasma (PRP) has also been widely researched on its expansive uses in different areas [14].
PRP is a platelet concentration obtained from blood by centrifugation. To be used at the surgical site, PRP should be stimulated to induce platelet degranulation and fibrin polymerization process, thus gaining a clot usually called Platelet Gel (PG). PRP is naturally diversified for various factors that exist in PRP preparation protocols including [1] the initial quantity of platelets, [2] the applications of anticoagulants, [3] the use of leukocytes, and [4] the inclusions of activators resulting in different biological outcomes. Aside from normally autologous state, and no danger of pathogen transmission or immunological dismissal, PRP contains the suitable extent of specific elements essential for wound healings [15]
The biological characteristics of PRP depend on the concentration in platelets; convenient preparations can aid PRP to give many growth factors (GFs) at high concentrations, including transforming growth factor-β, epidermal growth factor. Insulin-like growth factor also secreted in high concentration. Therefore, calibration of PRP is significant but the change in PRP concentration usually results in a low and unsteady repair effect in tissue regeneration. There are some protocols and refinement methods for PRP separation, various methods lead to various PRP components and concentrations as well as a different clinical result. A lot of researches elucidated that five times content of normal platelets can share in efficacious regeneration by PRP. And higher concentration does not motivate a better result [16].
Growth factors originated from PRP can participate in tissue regeneration, by facilitating cell migration, reproduction, Differentiation and synthesis of extracellular matrix [17].
However, the different GF concentrations may lead to different biologic effects, resulting in the fact that individual variation in GF levels should be taken into account for efficient translation of the biologic functions and calibrated use of PRP.
We can detect multiple GFs liberated from PRP, like the basic Fibroblast Growth Factor (bFGF), Platelet-Derived Growth Factor (PDGF), and Transforming Growth Factor-β (TGF-β); during the process of healing of trauma, numerous of these GFs have individual expression profile and are believed to play a significant function in the musculoskeletal regeneration. Generally, GFs are transmitted locally to the bone and soft tissue repair area with tissue-engineered scaffolds or dissolved in a fibrin-like packing [18].
In a study done by Wasterlain et al. to measure the impact of platelet-rich plasma injection on systemic growth factors level and to distinguish molecular markers to detect treated athletes. Serum bFGF, IGF-1, VEGF and levels are considerably elevated after PRP injection, assisting a potential ergogenic effect of PRP. An indirect sign for hGH. Platelet-rich plasma seems to trigger a rise in growth factors through activating biological pathways instead of by serving as a vehicle for the direct delivery of growth factors. Increased VEGF was noticed in all patients after PRP, and 88% of patients had increased VEGF at each time point from 3 to 96 hours after PRP, proposing that VEGF may be a sensible molecular marker to find out athletes recently treated with PRP. There is a statistically considerable elevation in circulating concentrations of VEGF, IGF-1, and bFGF after treatment with a single dose of leukocyte-rich PRP [19].
2 groups of Type-2 diabetes patients had been monitored in a private clinic, number 40 each group, with 30 females and 50 males. We measure fasting c-peptide before and after treatment with PRP injection and oral treatment in the form of dpp4 inhibitors and metformin in the first group.
In the second group, we give the patients oral treatment only in the form of dpp4 inhibitors and metformin.
The patients in the first group received prep 2 ml subcutaneous every week for 3 months plus ddp4 inhibitors and metformin and the second group received dpp4 inhibitors plus metformin.
Preparation of platelet rich plasma (PRP)
PRP is taken out from patients’ blood. A 20 cc venous blood draw yielding 3-4 ccs of PRP, we add an anticoagulant to prevent platelet activation prior to its use.
We prepare PRP by a process referred to as differential centrifugation, in which acceleration power is modified to precipitate specific cellular components with regard to various specific gravity.
PRP method
The blood was centrifuged with the use a soft spin. The floating plasma which contains platelets was transmitted to different sterile tube without adding anticoagulant; tube was centrifuged at a higher speed to obtain a platelet concentrate. The lower third of the tube is PRP and upper 2/3rd is Platelet Poor Plasma (PPP). Platelet pellets are created at the bed of the tube. PPP was removed and the platelet pellets suspended in a minimal volume of plasma 2-4 ml by gently shaking the tube. Pellets are mixed in the lower third of plasma to make the PRP.
The results are shown in Figures 1-3.
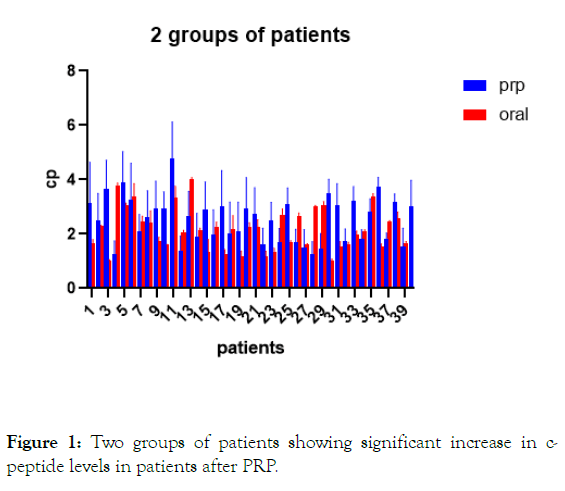
Figure 1: Two groups of patients showing significant increase in c-peptide levels in patients after PRP.
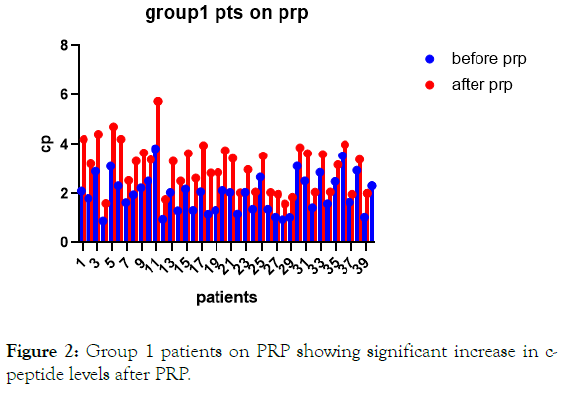
Figure 2: Group 1 patients on PRP showing significant increase in c-peptide levels after PRP.
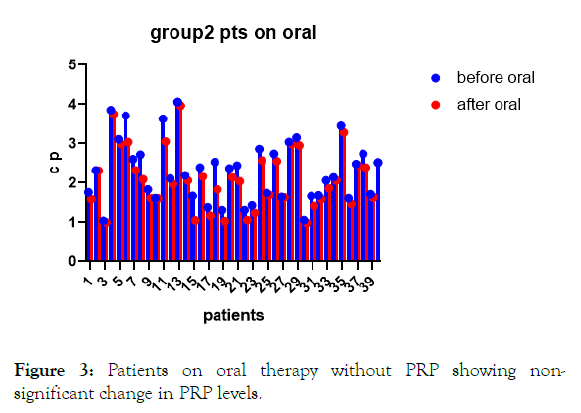
Figure 3: Patients on oral therapy without PRP showing nonsignificant change in PRP levels.
For analysis of the results of each group we used unpaired T-test (Tables 1-3).
Table 1: Group 1 on PRP and oral and group 2 on only oral.
| X | Group A | Group B | ||
|---|---|---|---|---|
| C-Peptide | PIP | Oral | ||
| X | Before | After | Before | After |
| 1 | 2.1 | 4.2 | 1.76 | 1.59 |
| 2 | 1.8 | 3.2 | 2.32 | 2.31 |
| 3 | 2.9 | 4.4 | 1.04 | 1 |
| 4 | 0.9 | 1.6 | 3.85 | 3.75 |
| 5 | 3.1 | 4.7 | 3.11 | 2.98 |
| 6 | 2.32 | 4.21 | 3.71 | 3.04 |
| 7 | 1.64 | 2.53 | 2.6 | 2.32 |
| 8 | 1.95 | 3.31 | 2.72 | 2.09 |
| 9 | 2.23 | 3.65 | 1.83 | 1.61 |
| 10 | 2.51 | 3.37 | 1.61 | 1.6 |
| 11 | 3.82 | 5.73 | 3.63 | 3.05 |
| 12 | 0.97 | 1.76 | 2.11 | 1.97 |
| 13 | 2.04 | 3.31 | 4.06 | 3.97 |
| 14 | 1.31 | 2.51 | 2.19 | 2.06 |
| 15 | 2.18 | 3.62 | 1.67 | 1.05 |
| 16 | 1.32 | 2.62 | 2.39 | 2.16 |
| 17 | 2.07 | 3.95 | 1.38 | 1.17 |
| 18 | 1.17 | 2.83 | 2.53 | 1.83 |
| 19 | 1.32 | 2.85 | 1.31 | 1.03 |
| 20 | 2.12 | 3.74 | 2.37 | 2.14 |
| 21 | 2.04 | 3.42 | 2.44 | 2.04 |
| 22 | 1.18 | 2.032 | 1.31 | 1.06 |
| 23 | 2.05 | 2.97 | 1.43 | 1.24 |
| 24 | 1.37 | 2.06 | 2.86 | 2.57 |
| 25 | 2.67 | 3.52 | 1.74 | 1.69 |
| 26 | 1.37 | 2.04 | 2.74 | 2.55 |
| 27 | 1.05 | 1.97 | 1.64 | 1.63 |
| 28 | 0.95 | 1.58 | 3.04 | 2.99 |
| 29 | 1.043 | 1.85 | 3.15 | 2.95 |
| 30 | 3.11 | 3.86 | 1.06 | 0.99 |
| 31 | 2.51 | 3.62 | 1.665 | 1.419 |
| 32 | 1.43 | 2.054 | 1.68 | 1.59 |
| 33 | 2.86 | 3.59 | 2.06 | 1.86 |
| 34 | 1.59 | 2.06 | 2.14 | 2.06 |
| 35 | 2.49 | 3.16 | 3.45 | 3.28 |
| 36 | 3.53 | 3.99 | 1.61 | 1.47 |
| 37 | 1.65 | 1.97 | 2.48 | 2.44 |
| 38 | 2.94 | 3.38 | 2.74 | 2.39 |
| 39 | 1.05 | 2.011 | 1.71 | 1.63 |
| 40 | 2.32 | 3.69 | 2.52 | 2.29 |
Table 2: Unpaired T-test column A after PRP vs column A before PRP, group 1 patients on PRP.
| Unpaired T-Test | |
|---|---|
| Table Analyzed | Data Taken |
| Column B | After prp |
| Column A | Before prp |
| p value summary | |
| p value | <0.0001 |
| Significantly different (p<0.05)? | Yes |
| One- or two-tailed p value? | Two-tailed |
| t value | 5.661 |
| df value | 78 |
| How big is the difference? | |
| Mean ± SEM of column A | 1.974 ± 0.12, n=40 |
| Mean ± SEM of column B | 3.073 ± 0.1525, n=40 |
| Difference between means | 1.099 ± 0.1941 |
| 95% confidence interval | 0.7122 to 1.485 |
| R squared (eta squared) | 0.2912 |
| F test to compare variances (p, DFn, Dfd) | |
| p | 1.614 |
| DFn | 39 |
| Dfd | 39 |
| P value | 0.1392 |
| P value summary | ns |
| Significantly different (p<0.05)? | No |
Table 3: Unpaired T-test column B after oral vs column A before oral, in the second group patients on oral hypoglycemic only (without PRP).
| Unpaired t Test | |
|---|---|
| Table Analyzed | Data Taken |
| Column B | oral after 3 months |
| Column A | oral before 3 months |
| p value summary | |
| p value | 0.2072 |
| Significantly different (p<0.05)? | No |
| One- or two-tailed p value? | Two-tailed |
| t value | 1.272 |
| df value | 78 |
| How big is the difference? | |
| Mean ± SEM of column A | 2.291 ± 0.1246, n=40 |
| Mean ± SEM of column B | 2.071 ± 0.1199, n=40 |
| Difference between means | -0.2199 ± 0.1729 |
| 95% confidence interval | -0.5641 to 0.1243 |
| R squared (eta squared) | 0.02032 |
| F test to compare variances (p, DFn, Dfd) | |
| p | 1.079 |
| DFn | 39 |
| Dfd | 39 |
| P value | 0.8125 |
| P value summary | ns |
| Significantly different (p<0.05)? | No |
Data analyzed
Sample size, column A 40.
Sample size, column B 40.
Increase in c-peptide levels in patients with PRP injection with dpp4 inhibitors and metformin. For 3 months with p-value less than 0.0001. With no statistical difference of c-peptide levels in patients on dpp4 inhibitors and metformin only with p-value 0.2072.
Data analyzed
Sample size, column A 40.
Sample size, column B 40.
Platelet rich plasma is a blood product which includes a great concentration of platelets within a little volume of plasma. PRP is used to hasten healing and repair [20].
PRP can be produced fluently, with minimum effort. Blood is centrifuged to split up plasma from red blood cells and then centrifuged to detach PRP from platelet-poor plasma. After that this concentrate must be stimulated with the addition of calcium, to give platelet gel. PRP to be considered of value for tissue repair should contain minimally a million platelets per microliter [21].
In this trial, the results show the effect of PRP injection on the regeneration of beta cell mass and increase of c-peptide secretion.
In this trial we discuss 2 points; first, the systemic effect of growth factors in platelet rich plasma and so it is evidenced from previous studies by Wasterlain et al., that systemic growth factors levels increase after PRP injection [19].
The second, the effect of increasing systemic levels of growth factors on pancreatic beta cells regeneration which is evinced in this trial by increasing c-peptide levels.
The GFs have a role in stimulating pancreatic β-cell proliferation. These molecules such as epidermal growth factor (EGF), which investigated for treating diabetes, can stimulate β- cell proliferation and insulin production.
The first limitation of the present trial is the small number of patients. While the inclusion of a few patients prohibits the extrapolation of our findings to the general population, the second limitation is whether these results will continue or will be transient.
Type-2 diabetes is a great health problem, and this trial aims to find new therapeutic options to cure.
Our findings indicate that PRP is a potential treatment for Type-2 diabetes. Further large-scale studies and clinical trials are required to verify our promising results.
As Growth factors (GFs) are considered as a natural biological mediators which control growth, differentiation, and have a role in the process of tissue reform and regeneration. The growth factors in platelet-rich plasma can induce beta cell regeneration and increase beta cell mass by stimulating β-cell neo-genesis and through ductal cell differentiation into β-cells which is detected by an increase in c-peptide levels which may add to Type-2 diabetes treatment.
Citation: Younis M (2019) Growth Factors in Platelet Rich Plasma can Regenerate Pancreatic Beta Cells in Type 2 Diabetes. Intern Med 9:304. doi:10.35248/2165-8048.19.9.304.
Received: 30-Jan-2019 Accepted: 23-Feb-2019 Published: 01-Mar-2019 , DOI: 10.35248/2165-8048.19.9.304
Copyright: © 2019 Younis M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.