Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Case Report - (2019)Volume 9, Issue 1
A 66-year-old male patient had been primarily treated for cutaneous lupus erythematosus 15 years ago. He received local corticosteroids and following systemic immunosuppressants: methylprednisolone (MP), chloroquine (removed due to retinal haemorrhage), cyclosporine A and azathioprine (both removed due to non-response). Due to polyarthralgia methotrexate as well as golimumab were started. Despite good response, both drugs had to be stopped due to development of hepatitis confirmed by liver biopsy (virus serological tests were negative).
Early after onset of a combination treatment with 1 g mycophenolate mofetil (MMF) per day orally and 10 mg/kg belimumab intravenously at weeks 0, 2, 4, followed by every 4 weeks, a marked decrease in liver enzymes could be detected. Due to insufficient response of lupus exanthema, hydroxychloroquine (HCQ) was added.
After 9 months of belimumab administration, the biologic therapy was switched to rituximab (1000 mg intravenously at weeks 0 and 2) in order to achieve a better control of cutaneous manifestation. Surprisingly, after the very first infusion a rapid and marked improvement of lupus exanthema was noticed. Such an improvement had not been experienced since 2003.
We could confirm an excellent response of systemic lupus erythematosus (SLE)-associated hepatitis as well as arthralgias to MMF and belimumab. Additionally, there was an excellent and prompt response of SLE-associated skin lesions to rituximab and MMF, despite the fact, that these drugs are still not approved for treatment of SLE. Verbal informed consent was obtained from the patient for the presentation of his disease course and images.
Lupus hepatitis; Systemic lupus erythematosus; Connective tissue disease; Immunosuppressants
In clinical practice we face many patients with different manifestations of systemic lupus erythematosus (SLE). Usually, they are being treated with immunosuppressive drugs to suppress systemic inflammatory processes. Corticosteroids are often drugs of first choice, since they act promptly, which may be beneficial in emergency cases, such as lupus nephritis [1] or severe neuropsychiatric conditions [2]. Antimalarials and methotrexate may help improve skin lesions and arthritis [3,4]. Several data show good response of lupus nephritis to cyclophosphamide, mycophenolate mofetil [5] or tacrolimus [6], used as induction treatment, and to azathioprine [7], used usually as maintenance treatment. Data support good efficacy of cyclosporine in lupus nephritis, hematologic involvement, arthritis, serositis or neurological manifestations [8].
However, the response of particular SLE presentations to these conventional disease modifying drugs may differ from patient to patient. Due to lacking data from large randomized trials the most immunosuppressants are not approved for treatment of SLE, with one exception – belimumab, a B-cell blocker, that showed to improve mucocutaneous and musculoskeletal manifestations predominantly [9]. Additionally, it exerts positive effects on lupus nephritis as well [10]. Another B-cell inhibitor, used to treat hematologic malignancies, rituximab, may be beneficial in SLE nephritis [11] and hematologic or neuropsychiatric conditions [12].
In this report, we present a mosaic picture of SLE and focus on skin, liver and joint involvement of SLE in our patient. The aim of our presentation is to show which combination regimens with biological drugs may be successfully used for particular manifestations of this connective tissue disease that do not respond to medications usually used in primary setting.
A male patient, born in 1951, developed at the beginning of 2003 an exanthema on his whole body (Figure 1). Skin biopsy showed cutaneous lupus erythematosus. Laboratory tests showed increased ANA-titre IgG 1:320 with positive SS-A subset; neither dsDNA-antibodies nor complement consumption could be detected. There were no changes in blood count. Initially, a treatment with corticoid cream and chloroquine was introduced. After 3 years, chloroquine had to be discarded due to retinal haemorrhage. The immunosuppressants cyclosporine A, followed by azathioprine, were started, both without response. Temporarily, chloroquine was re-started. In 2008, the patient developed polyarthralgia in wrists, elbows, knees as well as toes, associated with mild swelling. The dose of methylprednisolone (MP), continuously taken since 2003, had to be increased occasionally up to 40 mg per day. In 2009, pulmonary manifestation of lupus in terms of lupus pneumonitis was successfully treated with 75 mg prednisolone, and complete remission could be achieved within 5 months. In July 2010, a therapy with methotrexate (MTX) was introduced. After initial response of skin and joint symptoms, loss of its efficacy was suspected, and in January 2012 golimumab (GOL) was added to MTX. In July 2012, leflunomide (LEF) was added, however 6 weeks later, marked increase of liver enzymes was detected (gamma-glutamyl transferase (GGT) 42-fold of upper limit of normal (ULN)) and all three immunosuppressants (MTX, LEF and GOL) were stopped. However, no remission of pathological liver enzymes could be detected, and liver biopsy was performed. Histological analysis showed lobular and interface hepatitis (Figure 2). Since serological and PCR-tests for Ebstein-Barr-virus, cytomegalovirus as well as hepatitis A, B, C and antibodies against liver kidney microsomes and soluble liver antigen were negative, this finding was ascribed to liver involvement of lupus [13] though liver toxicity of the three mentioned immunosuppressants as well as of non-steroidal antiinflammatory drugs (NSAIDs) is also known [14-16]. At the beginning of 2013 the patient was referred to our department with above mentioned findings, co-medication (MP 15-20 mg per day, Calcium 1000 mg + Vitamin D3 800 IU per day, alendronate 70 mg weekly, gliclazide 30 mg on demand, lornoxicam 8 mg on demand, skin protection cream with sun protection factor 50 on demand) and following co-morbidities:
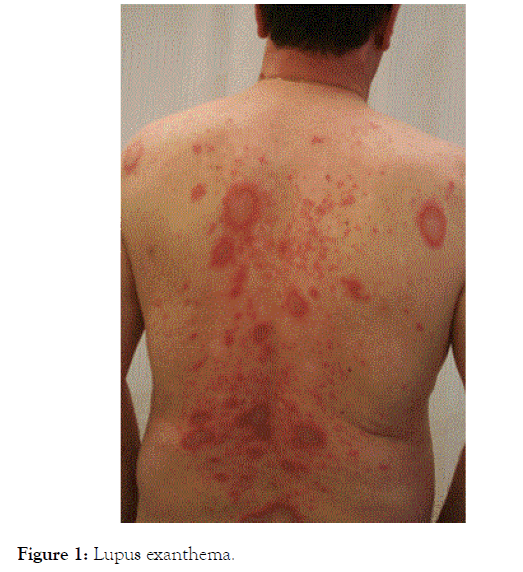
Figure 1. Lupus exanthema.
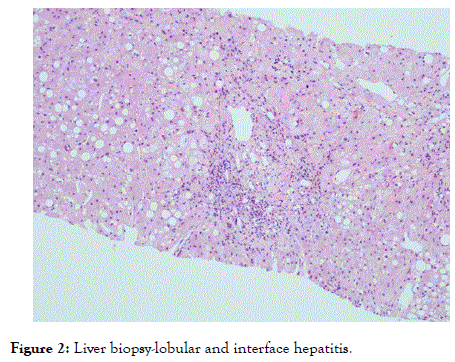
Figure 2. Liver biopsy-lobular and interface hepatitis.
Overweight with BMI 26.8 kg/m2
Diabetes mellitus type 2
Ex-nicotinism /stopped in January 2003/ (35 pack/years)
Osteochondrosis L4-S1 with disc bulging
Osteoporosis with vertebral fractures Th12-L2
History of wrist fracture (2000)
Cholecystolithiasis
Hypovitaminosis D
At first visit at our department the following status was obtained: large exanthemas on the trunk, tender wrists and toes (without swelling), positive squeeze test both on hands and feet, pain intensity based on visual analogue scale (VAS) 5, whereas the pain was present continuously. He was taking 20 mg and 15 mg MP alternately. Since the liver enzymes did not show a decreasing trend after cessation of the three immunosuppressants (MTX, LEF, GOL) through a period of 5 months, a combination treatment with 1 g mycophenolate mofetil (MMF) per day orally and 10 mg/kg belimumab at weeks 0, 2, 4, followed by every 4 weeks intravenously (due to extensive cutaneous manifestation on the whole body) was introduced in March 2013 [17,18]. Additionally, methylprednisolone was administered further at a dose of 10-20 mg per day (there had been no interruption in MP since 2003). The patient took 8-16 mg lornoxicam daily to ease his arthralgias. Due to recurrent exacerbations of exanthema a temporary increase of MP dose up to 40 mg per day was necessary. At that time, osteoporosis with a painful endplate impression of the 12th thoracic vertebra was diagnosed and treated conservatively and with alendronate orally; later on, a switch to annual intravenous administration of zoledronate was recommended. A marked decrease in liver enzymes could be detected, reaching normal values for transaminases and a GGT-level of only 3-fold above ULN within 7 months after onset of the combination therapy with MMF and belimumab [Table 1].
| Date of follow-up | ALT | GGT | PLT | Immunosuppressant |
|---|---|---|---|---|
| 29th June 2010 | 114 | 150 | 159 | NA |
| 4th June 2012 | 159 | 798 | 88 | MP+MTX+GOL |
| 5th Sept. 2012 | 194 | 1225 | 75 | MP+MTX+GOL+LEF |
| 2nd Oct. 2012 | 206 | 2178 | 134 | MP |
| 22nd Nov. 2012 | 204 | 2774 | 131 | MP |
| 17th April 2013 | 76 | 674 | 140 | MP+MMF+BEL |
| 25th July 2013 | 56 | 301 | 163 | MP+MMF+HCQ+BEL |
| 16th Oct. 2013 | 43 | 184 | 160 | MP+MMF+HCQ+BEL |
| 29th Jan. 2014 | 58 | 255 | 156 | MP+HCQ+RTX |
| 10th April 2014 | 80 | 350 | 177 | MP+HCQ+RTX |
| 10th July 2014 | 64 | 341 | 163 | MP+MMF+HCQ+RTX |
| 31th Oct. 2014 | 94 | 443 | 159 | MP+MMF+HCQ+RTX |
| 13th Feb. 2015 | 95 | 446 | 157 | MP+MMF+HCQ+RTX |
| 5th June 2015 | 81 | 406 | 159 | MP+MMF+HCQ+RTX |
| 2nd Feb. 2016 | 77 | 346 | 168 | MP+MMF+HCQ+RTX |
| 6th Sept. 2016 | 86 | 551 | 159 | MP+MMF+HCQ+RTX |
| 1st March 2017 | 71 | 345 | 180 | MP+MMF+HCQ+RTX |
| 9th June 2017 | 85 | 495 | 182 | MP+MMF+HCQ+RTX |
| 5th Sept. 2017 | 105 | 807 | 181 | MP+MMF+HCQ+RTX |
| 1st Feb. 2018 | 66 | 416 | 162 | MP+MMF+HCQ+RTX |
ALT: Alanine transaminase (normal range 10-50 U/l); BEL: Belimumab; GGT: Gamma-glutamyl transferase (normal range 10-66 U/l); GOL: Golimumab; HCQ: Hydroxychloroquine; LEF: Leflunomide; MMF: Mycophenolate mofetil; MP: Methylprednisolone; MTX: Methotrexate; NA: Not available; PLT: Platelets (normal range 150-400 G/l); RTX: Rituximab
Table 1: Course of pathological laboratory findings and immunosuppressive medication based on casual time selection (does not correspond with onset or withdrawal of particular drugs).
Due to insufficient response of lupus exanthema, hydroxychloroquine (HCQ) at a dose of 200 mg per day was added three months after onset of belimumab [19]. This led to a better control of arthralgias, which made possible a reduction of the MP dose and cessation of NSAIDs. Parallely to this combination treatment consisting of 4 immunosuppressants, a pneumocystis jirovecii prophylaxis with cotrimoxazole 3 times a week was introduced.
During belimumab administration, the response of exanthema was moderate, in the further course, however, missing with a tendency to worsening on the trunk, upper and lower extremities, even glans penis, where large circular lesions typical of systemic lupus erythematosus (SLE) - manifestations were found. After 9 months of belimumab administration (13 infusions), the biologic therapy was switched to rituximab in order to achieve a better response of cutaneous manifestation, as previously being observed [20]. At this time point, MMF was discarded due to better control of arthralgias (VAS 1-2), the dose of MP reduced to 2 mg per day and the dose of HCQ was increased to 400 mg per day. Rituximab was administered at a dose of 1000 mg intravenously at weeks 0 and 2. Surprisingly, after the very first infusion (within two weeks) a rapid and marked improvement of lupus exanthema was noticed (ca.70%). Such an improvement had not been experienced since 2003, though repeated MP pulses had been administered. Due to increase of pain intensity in peripheral joints, MP had to be raised to 4-8 mg per day and MMF re-introduced after 3 monthpause at a dose of 1 g per day [21]. This was increased 6 months later to 2 g per day due to persistent arthralgias. Cutaneous manifestations, especially minor lesions on glans penis, were treated successfully with tacrolimus ointment.
In further course, dermatological manifestations were limited to punctiform lesions and mild arthralgias were present occasionally only. Rituximab was administered every 6 months (2 infusions 2 weeks apart), 9 cycles up to now. Laboratory follow-ups showed stable blood count, renal function, no complement consumption, missing inflammatory parameters and anti-DNA antibodies, no signs of nephritic or nephrotic involvement. However, GGT tended to increase to approx. 7- fold of ULN, once reaching a 13-fold increase, which was detected once exceptionally [Table 1]. This has been observed after cessation of belimumab and during the combination of MMF + HCQ + rituximab and might be attributed either to withdrawal of belimumab or to adverse effects of the mentioned combination. The SLE disease activity index (SLEDAI) was in the range 0-6 all the time, never exceeding 6. The patient tolerated the above mentioned treatment regimens well, no marked adverse events have been reported.
We present a patient with multiorgan involvement of SLE: exanthema, hepatitis and articular manifestations. He had been initially treated by dermatologists due to skin lesions and antimalarials had been introduced in concordance with published data [19]. After development of joint manifestations, a rheumatologist was involved and introduced a combination therapy to achieve a better control of arthralgias (MTX+LEF +GOL), as usually performed in patients with rheumatoid arthritis [16,22]. The patient was, however, referred to our department after development of severe hepatitis that was interpreted as lupus hepatitis after histological analysis of the liver biopsy as a further possible presentation of SLE, known in the literature [13], though a toxic effect of previously used immunosuppressants was a matter of discussion as well [14-16].
There are rare data on treatment options for SLE-associated hepatitis. However, we could confirm an excellent response of this condition to MMF and belimumab, as previously published [17,18]. Additionally, we observed good response of joint pain to MMF in accordance with published data [23]. On the contrary, we could not confirm efficacy of a combination consisting of MMF, HCQ and belimumab in skin lesions, as published previously [18,19,24]. However, there was a prompt and excellent improvement of lupus exanthema after switching the biologic therapy from belimumab to rituximab, as described in the literature [25,26], despite the fact, that this drug is still not approved for treatment of SLE. The response persistence of skin manifestations may be attributed to combination treatment with MMF as well [24].
Currently, our patient is doing well and is being assessed at regular check-ups every 3 months and receives rituximab infusions every 6 months according to above mentioned regimen. He takes 4 mg MP, 2 g MMF and 400 mg HCQ daily and his disease exerts a stable course in terms of symptoms as well as laboratory parameters. Neither side effects nor infectious complications are being reported.
Based on our experience with this patient, we could recommend using immunosuppressive drugs that are not approved for treatment of above mentioned conditions due to missing data from large randomized trials. Naturally, such medications should be introduced after treatment failure or intolerance of approved drugs only. We want to encourage physicians to collect data from off-label use of immunosuppressants in connective tissue diseases to extend treatment possibilities in non-response or difficult patients.
The authors declare that there is no conflict of interest.
Citation: Psenak O, Studnicka-Benke A, Haufe H, Greil R (2019) Good Response of Lupus Hepatitis to Mycophenolate Mofetil And Belimumab as of Lupus Exanthema to Rituximab: A Case Report. Rheumatology (Sunnyvale). 9:247. doi: 10.35248/2161-1149.19.9.247
Received: 05-Jun-2019 Accepted: 25-Jun-2019 Published: 01-Jul-2019 , DOI: 10.35248/2161-1149.19.9.247
Copyright: © 2019 Psenak O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.