Drug Designing: Open Access
Open Access
ISSN: 2169-0138
ISSN: 2169-0138
Research Article - (2021)Volume 10, Issue 3
The aims of this study to formulate and evaluate the antimicrobial activity of Salvadora persica aqueous extract lozenges. S. persica aqueous extract lozenges were prepared by heating and congealing technique. Antimicrobial activities for S. persica aqueous extract and S. persica aqueous extract lozenges were determined using the well diffusion method. The formulation of S. persica lozenges was performed and its quality was evaluated. S. persica aqueous extract and S. persica aqueous extract lozenges were exhibited significant antibacterial and antifungal activities against clinical isolated oral pathogens. The highest antibacterial activity of; (85 mg/ml); 25 ± 0.78 mm, 30 ± 0.77 mm, 21 ± 0.83 mm and 18 ± 0.86 mm were obtained against E. coli, P. aeruginosa, S. aureus and S. mutans, respectively. Also, the antifungal activity of S. persica extract showed that the highest activity 12 ± 0.79 mm with concentration (85 mg/ml) against C. albicans. Moreover, S. persica extract lozenges were showed antimicrobial activities with the zone of inhibitions (85 mg/ml); 30 ± 0.83 mm, 20 ± 0.85 mm, 18 ± 0.78 mm and 10 ± 0.85 mm were obtained against E. coli, P. aeruginosa, S. aureus and C. albicans respectively. This study draws attention toward an easy and time saving formulation process of S. persica lozenges as suitable dosage form that may effectively control oral and throat pathogens.
Salvadora persica extract; Lozenges; Antimicrobial; Oral hygiene
Oral hygiene is the main portal for the body health most crucial component of our overall general health. It is a determinant factor for life quality as both are associated strongly [1,2]. The data mined about oral health from Global Burden of Disease Study in 2018 revealed that oral diseases are affect nearly 3.5 billion people globally [3], which can pose a serious health charge for many countries and affect individuals throughout their lifetime and influencing sleep, eating habits, and social factors [4]. Oral hygiene is diminished by many pathogenic microbes which affected the oral region by various damages that they may expand and cause serious disease [2]. Therefore, prevention and take control of oral pathogens is an important factor to maintain good oral hygiene [5].These opportunistic pathogens are frequently isolated from the oral cavity include Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus), Streptococcus pneumoniae (S. pneumoniae), Enterobacter cloacae (E. cloacae), Acinetobacter baumannii (A. baumannii), Stenotrophomonas maltophilia (S. maltophilia), and Streptococcus agalactiae (S. agalactiae) [6]. Several traditional medicinal plants have been proven for their activity and use in the prevention and treatment of different oral diseases [7]. Natural products have a unique diversity in chemical structure responsible for their pharmacological activities which in most cases are due to combined effects of the active constituents within the medicinal plant [8]. Historically, the first known natural product for oral hygiene tool was the chewing stick, the Miswak, derived mainly from Arak tree that grows in Saudi Arabia. It is obtained from (Salvadora persica L.) of the family Salvadoraceae, widespread in Middle Eastern, some Asian and African cultures traditionally utilize it as tooth-powder and tooth-cleaner. The World Health Organization (WHO) recommended the use of Miswak in 1986 and in 2000 in an international consensus report on oral hygiene [9,10]. S. persica has antibacterial activity [11]; antifungal properties [12]; antiviral activity [13]; dental plaque control [14]; antioxidant; antilipidemic and antidiabetic activity [15]. The major active constituents isolated from stem of S. persica for good oral hygiene are tannins, silica, alkaloids, volatile oil, sulphur monocline, organic sulphur compounds, chlorides, calcium, β-sitosterol and saponins (Table 1). Lozenges are dosage forms manufactured in a simple way, which can deliver an active ingredient as they disintegrate slowly in the oral cavity [16]. Lozenges considerable has advantages over oral sprays or gargles, which provide fast, effective and sustainable delivery of active ingredients representing a good vehicle for active constituents, therefore highlighting their potential benefits for mouth and throat medication with palatable mean and high patient adherence [17].
| Active constituents | Pharmacological activity |
|---|---|
| Tannins (tannic acid) | Reduces plaque and gingivitis. |
| Silica | An abrasive material to remove stains on teeth. |
| Alkaloids (salvadorine) | Bactericidal effect and stimulate the gingiva. |
| Essential oils | Antimicrobial activity and stimulates the flow of saliva, which acts as a buffering agent. |
| Benzylisothiocyanate | Chemo-preventive, antiviral, antibacterial and anti-fungal agents. |
| Vitamin C | It helps in tissue healing and repair. |
| Fluoride | Anti-decay effects. |
| Chloride | Inhibits calculus formation and help in removing stains from the teeth. |
| Calcium | Its saturation of saliva inhibits demineralization and induces the re-mineralization of tooth enamel. |
| Sulfur | Bactericidal effect. |
Table 1: The active constituents from stem of S. persica and their pharmacological benefits for oral hygiene.
Therefore, this study aimed to formulate S. persica aqueous extract in form of lozenges and evaluate their formulation properties as well as antimicrobial activity against different bacterial species and fungi, specifically Candida albicans. As we are targeting oral hygiene the choice of lozenges will be more reasonable compared to the other solid dosage forms [18-28].
Plant materials
S. persica sticks were collected from Al Azazi area, Gezira state, Sudan. The studied plant part was identified and authenticated by Medicinal and Aromatic Plants Research Centre, Faculty of Pharmacy, University of Gezira and Agricultural Research Corporation (ARC), Wad Medani, Sudan.
Preparation of plant extract
The collected sticks were cut into small pieces (0.5 cm-1.0 cm) and dried in an air-open shade, then milled into coarse powder using electrical blender (Moulinex Blender the genuine 400 W, France). The powdered plant material (286.4 g) was macerated in 1.30 liter of cold distilled water at refrigerator for 72 hours with occasional shaking; then filtered using Whatman filter paper (Whatman® glass microfiber filters, 90 mm Grade GF/B; Aldrich, Germany) twice. Filtrate was evaporated using rotary evaporator at 60°C (Heidolph, Germany) until dryness to yield a green mass, followed by freeze-drying (LYO GT2, SRK-Systemtechnik GmbH, Germany) and was stored until use.
Qualitative phytochemical screening
S. persica extract was subjected to phytochemical screening for their different constituents such as; carbohydrates and/or glycosides, alkaloids and/or nitrogenous compounds, flavonoids, tannins and/or phenolic compounds, sterols and/or triterpenes, saponins and sulfur compounds [29].
Formulation of S. persica hard candy lozenges
Two standard model of hard candy lozenges formula based on different excipients were prepared by heating and congealing technique [30]. The compositions of model formulations are given in Table 2. For formula I, the candy base was prepared by dissolving of fine powder sugar in one-third amount of distilled water in candy base cooker. This had been continued till the temperature reached 200°C. Liquid glucose was then poured slowly and temperature lowered to 150°C in order to add the methyl cellulose till the mixture became candy mass. Sodium citrate powder and amaranth red (coloring agent) were added to the candy mass with continuous stirring. Then the mass was removed from the cooker and then menthol powder flavor and S. persica extract were added and mixed thoroughly. Finally, the mass was poured in pre-calibrated mold and left for 15 minutes to congeal. The same procedure was repeated for formula II with sodium carboxymethyl cellulose (NaCMC) instead of methyl cellulose (MC) (Table 2).
| Ingredients (Single lozenge) | Formula I | Formula II |
|---|---|---|
| Sugar | 67.5 g | 67.5 g |
| Liquid glucose | 28.1 g | 28.1 g |
| Methylcellulose 1% (MC) | 2 g | 0 |
| Sodium carboxymethylcellulose 1% (CMC) | 0 | 2 g |
| Acidulent (Sodium Citrate) | 1 g | 1 g |
| Flavor (Menthol) | 0.67 g | 0.67 g |
| Color (Amaranth) | 0.03 g | 0.03 g |
| S. persica extract | 75 mg | 75 mg |
Table 2: The compositions of S. persica extract hard candy lozenges standard formulation.
Quality control analysis of S. persica extracts lozenges
Moisture content analysis: Gravimetric method for moisture analysis was followed, in which sample (1 g) will be weighed and placed in vacuum oven at 60°C-70°C for 12-16 hours. Final weight will be subtracted from initial and differences in moisture content were calculated [31].
Determination of weight variations and diameter: The average weight of the lozenges was determined by individually weighing of 20 lozenges of each formulation using an analytical balance (W3100A-210, Accuris™, USA), individual weight was compared to the average weight and subsequently the mean weights and the respective standard errors were calculated. Also, the diameter was measured for 20 lozenges [32].
In vitro dissolution experiment: In vitro dissolution test was conducted based on United State Pharmacopeia method II [33] with little modifications using USP apparatus II (RC-6 Dissolution tester, Guoming® , China). Artificial saliva pH 6.8 at 37 ± 0.5°C was taken as dissolution media. Each lozenge was immersed in 1000 mL vessel and the rotation speed was set to 100 rpm. At different time intervals (5, 10, 15 and 20 minutes) a 5 mL sample was withdrawn and replaced with 5 ml fresh artificial Saliva as shows in Table 3. UV/Visible spectroscopy was used to determine the absorbance at wavelength 275 nm (7205 UV/Visible spectrophotometer, Jenway, UK). The samples were then determined spectrophotometrically based on the λmax [34].
| Ingredients | Quantity |
|---|---|
| Sodium chloride | 8.000 g |
| Potassium dihydrogen phosphate | 0.190 g |
| Sodium hydrogen phosphate dihydrate | 2.984 g |
| Phosphoric acid | ad pH 6.80 |
| Demineralized water | ad 1 L |
Table 3: The composition of artificial saliva.
Antimicrobial activity
Tested microorganisms: Tested microorganisms were clinically isolated microorganisms including four bacterial strains; Escherichia coli (ML-UG/B130024), Pseudomonas aeruginosa (ML-UG/B130025), Staphylococcus aureus , streptococcus mutans (ML-UG/B130028) and one fungal strain Candida albican (ML-UG/F130001); and identified by the Microbiology Department Laboratory at Faculty of Pharmacy, University of Gezira, Wad Medani, Sudan.
Microorganisms growth condition and antimicrobial assay: The antibacterial and antifungal activities of S. persica aqueous extract and S. persica aqueous extract lozenges were determined using the well diffusion method. Petri plates containing 20 ml of, nutrient agar for bacteria (Darwin Biological, UK) or malt extract for fungus (Himedia, India), agar medium was seeded with 1-3 days cultures of microbial inoculums. Wells (6 mm in diameter) were cut off from agar and 50 μl of the samples in different concentrations and standard antibiotics were tested and then incubated at 37°C for 24-48 hours (bacterial strains) and for 3-5 days (fungal strain). The antibacterial and antifungal activities were determined by measurement of the diameter of the inhibition zone surrounding the well [35].
Data analysis
All the obtained data were expressed as means ± Standard Error of Means (SE) and analyzed using analysis of variance (ANOVA). Comparisons with the control groups were made using One-way ANOVA. The level of statistical significance was set at p<0.05
Extraction and phytochemical screening
S. persica stick aqueous extraction process was yielded 34.8 g of dry green extract (12.15% w/w). The qualitative preliminary phytochemical screening indicated that S. persica extract contain tannins and/or phenolic compounds, sulfur compounds, carbohydrates and/or glycoside and Saponins (Table 4). On the other hand, alkaloids and/or nitrogenous compounds, flavonoids, and sterols and/or triterpenes were absent. These phytochemical results can support possible presence of S. persica extract biological activities [11,18,28].
| Test | Result |
|---|---|
| Sulfur compounds | + |
| Tannins | + |
| Glycoside | +/- |
| Alkaloids | - |
| Flavonoids | - |
| Flavones | - |
| Saponins | - |
*Key: (+) =Present; (-) =Absent.
Table 4: The phytochemical screening results of S. persica extract.
Formulation of S. persica lozenges
All the formulated lozenges were showed good physical appearance (Figure 1), thereby attractive and unpleasant taste masking were found in S. persica lozenges. Based on the general physical texture, lozenges contain MC were more solid compared to that one containing NaCMC which lead to more chewable lozenge as the salt act as hygroscopic agent resulting in increased moisture content. The addition of hydrocolloid; NaCMC and MC; directly affected the formulated lozenges in term of solid nature, chewing capability and extended the release of extract (Figure 1) [36].
Figure 1: S. persica extract lozenges physical appearance.
Quality control analysis of S. persica extract lozenges
The average weight of S. persica lozenges was found (3.228 g ± 0.206 g), while the mean of lozenges diameter was found (16 mm ± 0.75) as shown in Table 5, which found to be within the standard limits.
| S. NO. | Weight of lozenges in grams ± SE | Diameter in cm ± SE |
|---|---|---|
| 1 | 3.2100 ± 0.0008 | 1.6 ± 0.28 |
| 2 | 3.1539 ± 0.059 | 1.5 ± 0.35 |
| 3 | 3.3539 ± 0.07 | 1.6 ± 0.34 |
| 4 | 3.5746 ± 0.010 | 1.7 ± 0.28 |
| 5 | 3.0143 ± 0.014 | 1.5 ± 0.35 |
| 6 | 3.0646 ± 0.017 | 1.6 ± 0.34 |
| Average | 3.2285 | 1.6 |
| SD | 0.2069 | 0.075 |
*Abbreviation: SE: Standard Error; SD: Standard Deviation
Table 5: Weight and diameters of S. persica lozenges.
The moisture content of all the formulations found to be below 2% ± 0.3 (Table 6). This is due to suitable amounts of water soluble polymer and less water uptake. For in vitro drug release, randomly selected six lozenges were tested. The percentage release of S. persica extract from lozenges was presented in Table 7 and Figure 2. The results obtained were showed extended release of the S. persica extract over a period of 20 minutes. All the six lozenges at the first 5 minutes were released about 11%-14% ± 0.16%-0.6% of the total content. The cumulative amount was released increased gradually up to 100% after 20 minutes. The extend release behavior mostly is due to the presence of the hydrocolloid polymer in the formula. The results were obtained by Abdoun and Alenizi, 2019 when formulated a clotrimazole in polymer showed that the drug is released in 30 minutes was 97.45% ± 0.7% and 91.76% ± 0.83% from guar gum methyl cellulose lozenges respectively, while 97.31% drug release in 7 minutes from lozenges containing no polymer [37].
| S. No. | Weight before (g) ± SE | Weight after (g) ± SE | % of weight loss |
|---|---|---|---|
| 1 | 3.2100 ± 0.02 | 3.1600 ± 0.01 | 1.56% |
| 2 | 3.1539 ± 0.03 | 3.1355 ± 0.01 | 0.58% |
| 3 | 3.3539 ± 0.11 | 3.3345 ± 0.11 | 0.58% |
| 4 | 3.5746 ± 0.10 | 3.5542 ± 0.10 | 0.57% |
| 5 | 3.0143 ± 0.03 | 2.9950 ± 0.02 | 0.64% |
| 6 | 3.0646 ± 0.02 | 3.0410 ± 0.02 | 0.77% |
*Abbreviation: SE: Standard Error.
Table 6: S. persica lozenges moisture content (Formula II).
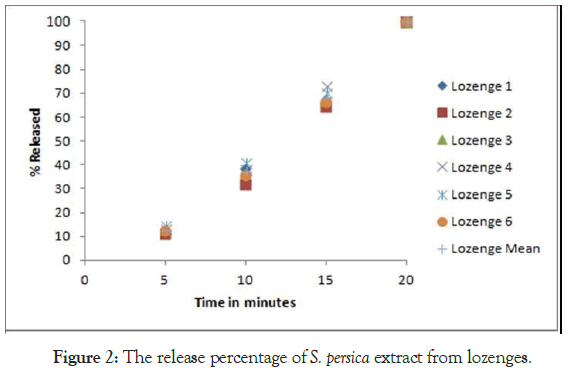
Figure 2: The release percentage of S. persica extract from lozenges.
| Time (Min) | % Release ± SE | |||||
|---|---|---|---|---|---|---|
| Lozenge 1 | Lozenge 2 | Lozenge 3 | Lozenge 4 | Lozenge 5 | Lozenge 6 | |
| 5 | 11.58 ± 0.2 | 11.18 ± 0.2 | 13 ± 0.16 | 13.32 ± 0.6 | 14.53 ± 0.6 | 12.74 ± 0.16 |
| 10 | 38.71 ± 0.13 | 32.35 ± 1.68 | 36.94 ± 1.98 | 38.46 ± 0.12 | 40.91 ± 1.90 | 35.71 ± 1.68 |
| 15 | 67.74 ± 0.35 | 64.71 ± 0.98 | 67.03 ± 0.36 | 73.08 ± 1.31 | 70.45 ± 1.32 | 66.67 ± 0.98 |
| 20 | 100 | 100 | 100 | 100 | 100 | 100 |
*Abbreviation: SE: Standard Error
Table 7: The release percentage of S. persica extract from lozenges (Formula II).
Antimicrobial assay
The antibacterial and antifungal activities of S. persica extract and S. persica extract lozenges (Formula II) were identified using welldiffusion method. The antibacterial activity of S. persica extract showed that the highest zone of inhibitions (85 mg/ml); 25 ± 0.78 mm, 30 ± 0.77 mm, 21 ± 0.83 mm and 18 ± 0.86 mm were obtained against E. coli, P. aeruginosa, S. aureus and S. mutans, respectively (Table 8 and Figure 3), which significantly higher than zone of inhibition of antimicrobial standards (cefuroxime and chlorhexidine) against bacterial strains. Also, the antifungal activity of S. persica extract showed that the highest activity 12 ± 0.79 mm with concentration (85 mg/ml) against C. albicans. The antimicrobial activity of S. persica which from this study was higher than that study investigated the effect aqueous extracts of S. persica against oral pathogens; with zone of inhibitions (200 mg/ml); 10.8 mm, 18.4 mm, 19.3 mm and 12.4 mm were obtained against P. aeruginosa, S. aureus, S. mutans, and C. albicans respectively [38]. On the other hand, S. persica extract lozenges showed that the highest zone of inhibitions (75 mg/ml); 30 ± 0.83 mm, 20 ± 0.85 mm and 18 ± 0.78 were obtained against E. coli, P. aeruginosa and S. aureus respectively (Table 8 and Figure 3), which higher than zone of inhibition of antimicrobial standards (cefuroxime and chlorhexidine) against the same bacterial strains.
| Sample | Concentration | E. coli | P. aeruginosa | S. aurues | S. mutans | C. albicans |
|---|---|---|---|---|---|---|
| Zone of inhibition (mm) ± SE | ||||||
| S. persica extract | 12.5 mg/ml | 13 ± 0.19 | 18 ± 0.63 | 11 ± 0.35 | 10 ± 0.75 | -ve |
| 25 mg/ml | 15 ± 0.18 | 20 ± 0.88 | 20 ± 0.66 | 13 ± 0.79 | -ve | |
| 50 mg/ml | 23 ± 0.85 | 25 ± 0.81 | 21 ± 0.73 | 13 ± 0.81 | 10 ± 0.45 | |
| 75 mg/ml | 25 ± 0.88 | 26 ± 0.75 | 20 ± 0.89 | 15 ± 0.65 | 12 ± 0.60 | |
| 85 mg/ml | 25 ± 0.78 | 30 ± 0.77 | 21 ± 0.83 | 18 ± 0.86 | 12 ± 0.79 | |
| S. persica lozenges | 75 mg/ml | 30 ± 0.83 | 20 ± 0.85 | 18 ± 0.78 | 15 ± 0.88 | 10 ± 0.85 |
| Cefuroxime (S) | 75 mg/ml | 15 ± 0.55 | 19 ± 0.80 | 20 ± 0.86 | 16 ± 0.86 | - |
| Nystatin (S) | 1003 IU/ml | - | - | - | - | 25 ± 0.73 |
| Chlorohexidine (S) | 1% | 16 ± 0.82 | 20 ± 0.87 | 26 ± 0.59 | 17 ± 0.43 | 10 ± 0.82 |
*Abbreviation: SE: Standard Error; -ve: Negative
Table 8: Antimicrobial activity zone inhibitions of S. persica extract and S. persica extract lozenges.
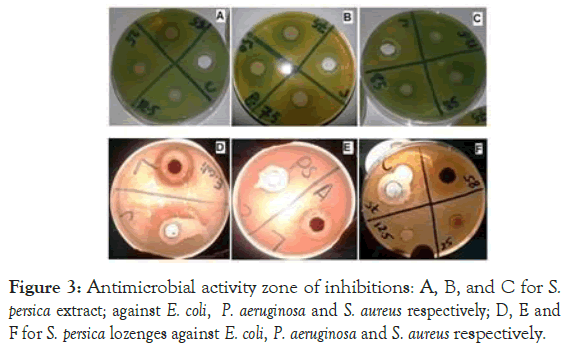
Figure 3: Antimicrobial activity zone of inhibitions: A, B, and C for S. persica extract; against E. coli, P. aeruginosa and S. aureus respectively; D, E and F for S. persica lozenges against E. coli, P. aeruginosa and S. aureus respectively.
This study confirmed the beneficial effect of S. persica on oral health that may due to the presence of important compounds, which have antimicrobial effect. Fluoride was reported to inhibit the growth of bacteria [21,26]. Tannins were found to reduce gingivitis [18,19], silica and chloride removing stains and helpful in tooth whitening [20,23,27], and vitamin C help in tissue healing and repairing [21]. Moreover, the essential oils beside antimicrobial activity have bitter taste which stimulates the flow of saliva and acts as a buffering agent [22,23].
Results of the current study revealed the formulation of S. persica aqueous extract lozenges were successful prepared with good quality. S. persica aqueous extract and S. persica aqueous extract lozenges were exhibited potent antibacterial and antifungal activities against clinical isolated oral pathogens. This study draws attention toward an easy and time saving formulation process of S. persica lozenges as suitable dosage form that control oral and throat infections with rapid onset of action, economic and good patient compliance.
There has been no significant financial support for this work that could have influenced its outcome.
The authors declare that they have no competing interest.
The authors would like to extend their sincere gratitude to Faculty of Pharmacy, University of Gezira for support.
Citation: Hussein EF, Abdelgadir WA, Osman RM, Waddad AY, Abdelgadir AA (2021) Formulation and Antimicrobial Evaluation of Miswak (Salvadora persica L.) Chewing Stick Aqueous Extract Lozenges. Drug Des. 10:182.
Received: 26-Mar-2021 Accepted: 09-Apr-2021 Published: 16-Apr-2021 , DOI: 10.35248/2169-0138.21.10.182
Copyright: © 2021 Hussein EF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.