Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2019)Volume 8, Issue 1
Introduction: A prostate cancer case in sub-Saharan Africa is rising. In Sudan, prostate cancer ranks third at after breast cancer. The nuclear deoxyribonucleic acid content has been shown to be of great prognostic value in prostate tumors. In the present work, we focus on semi-quantification of nuclear deoxyribonucleic acid in prostate cancer compared to prostatic hyperplasia using the Feulgen reaction technique.
Methods: Paraffin wax sections were selected from patients previously diagnosed with prostatic hyperplasia and prostatic cancer. Clinic pathological data of patients were collected from the records of the archive of the National Health Laboratory Khartoum Sudan. From all cases, two sections were taken. One section was stained with Hematoxylin and eosin stain to confirm the diagnosis, while the other paraffin section was used for deoxyribonucleic acid demonstration using Feulgen reaction.
Results: A total of 46 patients with a clinical and pathologic diagnosis of prostatic tumors were enrolled in this study; 23 (50.1%) had high-grade prostatic adenocarcinoma, 11 (23.9%) had moderate grade prostatic adenocarcinoma, two (4.3%) had low-grade prostatic adenocarcinoma and 10 (21.7%) had benign hyperplasia. The significant differences in deoxyribonucleic acid staining intensities were observed in high-grade prostatic adenocarcinomas. The deoxyribonucleic acid staining intensities of moderate and high-grade prostatic adenocarcinomas were significantly higher than those of benign hyperplasia (P<0.000).
Conclusion: Deoxyribonucleic acid detection using Feulgen reaction may provide valuable prognostic information in prostate cancer, with potential clinical implications in patient management, active research on this topic should be a high priority objective.
Prostatic hyperplasia; Prostate cancer; Feulgen reaction
Cancer is becoming a global health problem [1] and the number of prostate cancer cases in sub-Saharan Africa is rising [2]. Prostate cancer in Sudan is number three after breast, leukemia, and lymphoma [3]. It’s one of the leading causes of death in the United States [4]. Recognizable changes in nuclear shape and size might provide a useful biomarker. Indeed, nuclear enlargement, irregularity, hyperchromasia, and prominence of nucleoli are among the hallmarks used to distinguish high-grade prostatic cancer from prostatic hyperplasia [5], computer-assisted nuclear morphometry still has the abundant undeveloped potential for the discovery of useful biomarkers in prostate cancer research [6]. The feulgen reaction has been proposed by Robert Feulgen and Heinrich Rossenbeck for the identification of DNA [7]. It has been considered an objective method with high interobserver reproducibility and a useful tool for diagnostic and prognostic purposes [8]. Its advantages over flow cytometry include the possibility of long-term storage of the biological material and feasible determination of very small amounts of DNA [9]. The Feulgen reaction can identify nuclear phenotypes that discriminate regions corresponding to heterochromatin and euchromatin domains [10]. DNA in cells staining based on the reaction of Schiff reagents with aldehyde groups procreate in the deoxyribose molecules by hydrochloric acid hydrolysis. The staining intensity is proportional to the DNA concentration [7]. Morphologic grading of prostate cancer is highly subjective and does not always reflect the true biologic malignancy of the tumor [11]. Therefore, an accurate, and preferably objective, a method for malignancy grading of prostatic carcinoma is highly desirable. Nuclear DNA content has been shown to be of prognostic value in prostate tumor [12,13] and in several other tumor type tumors [14,15]. Feulgen DNA reaction can be performed on Hematoxylin and Eosin destined cytological specimens [16]. It ’ s possible to perform detailed retrospective studies based on Feulgen-stained archival slides [17]. In the present work, we focus on semi-quantification of nuclear DNA in prostate cancer compared to prostatic hyperplasia using the Feulgen reaction technique.
This study was conducted at Faculty of Medical Laboratory Sciences, histopathology department Sudan University for Science and Technology and National Health Laboratory (NHL) in Khartoum state Republic of Sudan (May 2015-June 2016). Paraffin wax sections were selected from patients that were previously diagnosed as prostatic hyperplasia and prostatic cancer at the National Health Laboratory. Clinic pathologic data of patients were collected from the records of the archive of the National Health Laboratory; their ages were ranged from 40 to 90 years. Tiny biopsies and the focal nodular prostate cancer were excluded. Forty-six paraffin blocks were included in this study; thirty-six specimens were prostate cancer and ten specimens as prostatic hyperplasia. The tissues were cut into 4 μm thick sections and mounted on microscope slides. All sections were incubated in the oven at 60ºC for one hour, then in three changes of xylene two minutes in each to remove the wax and were treated with three changes of absolute ethyl alcohol each for two minutes then rehydrated through gradual ethyl alcohol and placed in water. Form all cases two section were taken, one section was stained with Hematoxylin and Eosin stain as described by Fischer et al. to confirm the diagnosis [18]. The other paraffin section used for DNA demonstration uses the Feulgen reaction. In which sections were placed in normal hydrochloric acid (N-HCl) at room temperature for one minute, then treated with preheated (NHCl) at 60°C for ten minutes, again sections were rinsed in (NHCl) at room temperature for one minute, after that sections transferred to Schiff’s reagent for 45 minutes, then rinsed in three changes of bisulphite solution two minutes each, then rinsed in water and counter stained in 1% light green SF for one minute, finally sections were dehydrated in alcohol cleared in xylene and mounted in D.P.X. All sections were stained in the same batch to eliminate inter-batch variation. The slides were read by a pathologist who remained blind about the clinical characteristics and histopathology on the arms of the study.
Data collection was carried out with SPSS version 16.0. The Student t-test and Fisher exact test were used to evaluating the qualitative and quantitative variable differences, respectively. The correlation between prostatic tumor and DNA content status was determined by Pearson correlation analysis. Each test was two-sided, and a p-value <0.05 was considered statistically significant.
The mean age of study group was 64 years and median 65 it was ranged from 40 years to 90 year main histological grads of 46 patients studied with ages between 40 and 90 years, are presented on (Table 1).
| Age groups Grade |
40-50 | 51-60 | 61-70 | 71-80 | 81-90 | Total |
|---|---|---|---|---|---|---|
| No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | |
| Hyperplasia | 2 (4.3 %) | 4 (8.7 %) | 3 (6.5 %) | 1 (2.2 %) | 0 (0 %) | 10 (21.7 %) |
| Low grade | 0 (0 %) | 0 (0 %) | 2 (4.3 %) | 0 (0 %) | 0 (0 %) | 2 (4.3 %) |
| Moderate grade | 1 (2.2 %) | 0 (0 %) | 7 (15. 2 %) | 3 (6.5 %) | 0 (0 %) | 11 (23.9 %) |
| High grade | 1 (2.2 %) | 4 (8.7 %) | 11 (23.9 %) | 5 (10.9 %) | 2 (4.3 %) | 23 (50 %) |
| Total | 4 (8.7 %) | 8 (17.4 %) | 23 (50 %) | 9 (19.6 %) | 2 (4.3 %) | 46 (100 %) |
Table 1: Grades of prostate tumors and age groups in years.
Staining intensity of Feulgen reaction for DNA demonstration in paraffin section was compared between prostatic adenocarcinoma and benign prostatic hyperplasia. A total of 46 patients with a clinical and pathologic diagnosis of prostatic tumors were enrolled in this study; 23 (50.1%) had high-grade prostatic adenocarcinoma, 11 (23.9%) had moderate grade prostatic adenocarcinoma, two (4.3%) had low-grade prostatic adenocarcinoma and 10 (21.7%) had benign hyperplasia.
A comparison between and the detailed staining result of Feulgen reaction for DNA in different grade of prostatic adenocarcinoma and benign prostatic hyperplasia among study group are shown in (Table 2).
| Prostatic tumor grade | Hyperplasia | Low grade | Moderate grade | High grade | Total |
|---|---|---|---|---|---|
| Feulgen reaction for DNA | No (%) | No (%) | No (%) | No (%) | No (%) |
| Negative | 1 (2.2 %) | 0 (0.0%) | 3 (6.5 %) | 3 (6.5 %) | 7 (15.2%) |
| Weakly positive (+) | 5 (10.9%) | 2 (4.3 %) | 1 (2.2 %) | 1 (2.2%) | 9 (19.6 %) |
| Moderately positive (++) | 3 (6.5 %) | 0 (0.0%) | 5 (10.9%) | 2 (4.3 %) | 10 (21.7 %) |
| Strong positive (+++) | 1 (2.2 %) | 0 (0.0 %) | 2 (4.3 %) | 10 (21.7%) | 13 (28.3 %) |
| Strongly positive (++++) | 0 (0.0 %) | 0 (0.0 %) | 0 (0.0) | 7 (15.2 %) | 7 (15.2 %) |
| Total | 10 (21.7 %) | 2 (4.3 %) | 11 (23.9 %) | 23 (50.1 %) | 46 (100 %) |
| p: Value with chi-square test | p=0.000 | p=0.000 | p=0.000 | p=0.000 |
Table 2: Feulgen reaction and prostatic tumor grade.
The significant differences in DNA staining intensity using Feulgen reaction were observed in high grads prostatic adenocarcinoma (Figure 1).
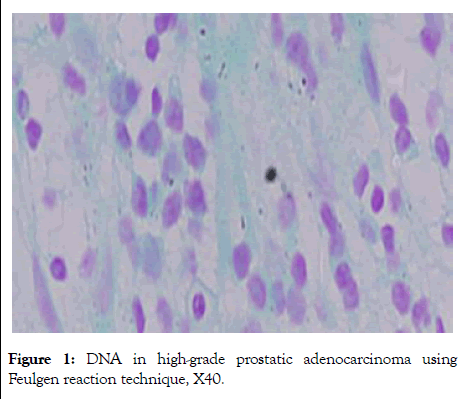
Figure 1: DNA in high-grade prostatic adenocarcinoma using Feulgen reaction technique, X40.
However, the majority of benign hyperplasia and low-grade prostatic adenocarcinoma cases shows weak positivity (Figures 2 and 3). The staining intensity of DNA of moderate and high grads prostatic adenocarcinoma was significantly higher than those of benign hyperplasia (all p<0.000).
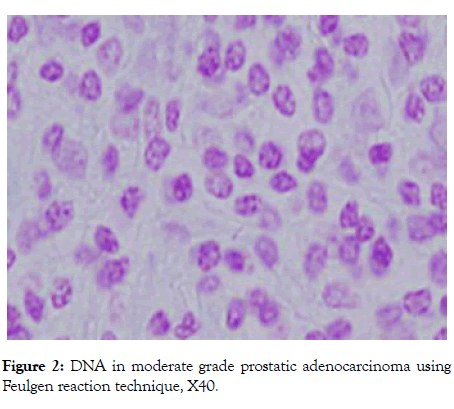
Figure 2: DNA in moderate grade prostatic adenocarcinoma using Feulgen reaction technique, X40.
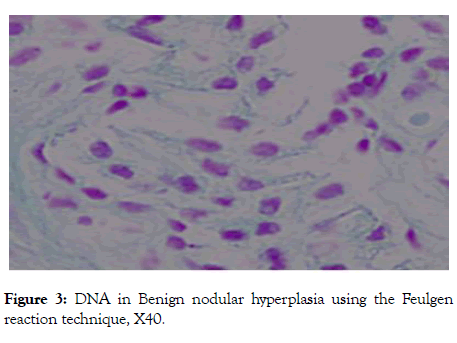
Figure 3: DNA in Benign nodular hyperplasia using the Feulgen reaction technique, X40.
In the current study, the relationship between DNA positivity using Feulgen reaction and degree of malignancy was investigated in prostatic hyperplasia and carcinoma. This technique conveniently allows retrospective studies. DNA data can be correlated directly to the known clinical course of the tumor disease. This circumvents the need for long-term prospective investigations. With the use of criteria for ploidy level determinations. The mean age of our study group was 64 years This confirmed with previous studies as they found nearly similar findings [19,20]. In the present study, we focus on semiquantification of nuclear DNA patterns as a biomarker for the early stage of pre-neoplastic change in the benign prostatic epithelium, a stage associated with precancerous changes, validation of such a biomarker could lead to both clinical and research applications. Clinically, DNA detection could be used to predict the presence of cancer. Prostate cancer is typically diagnosed by needle biopsy, using Prostate Specific Antigen as an indicator for biopsy and the absence of any imaging method for visualizing lesions [21]. Earlier similar studies, shown that DNA positivity increases as the tumor progresses from benign to cancer conditions. In renal, lung, bladder tumors and prostate [22-26]. In high-grade prostatic adenocarcinoma cases, other studies also confirm our finding that a highly reactive DNA was associated with a high histologic grade [21]. This study suggests that DNA demonstration with Feulgen reaction could be used as a marker of this change in biological behavior. Histological grads shows a statistically significant increase in staining intensity (p<0.005) as the disease progressed from benign hyperplasia to prostatic cancer. Kallakury B et al., [27] Wang N [28] and Santourlidis et al. [29], found that DNA was generally low in benign prostatic hyperplasia although it was higher in different grades of prostate cancer. Our findings are similar.
Accurate preoperative prediction of progression in localized tumor and recurrence is urgently needed to select the right patients for curative therapy, enable appropriate counseling and select patients for adjuvant therapy and has been the goal of many studies over the past two decades. This study has shown that DNA positivity can be taken as independent predictors of progression and recurrence of prostate cancer. Data on repeat biopsy in studies similar to ours are scanty.
Our results suggest that Deoxyribonucleic acid demonstration using Feulgen reaction may provide valuable prognostic information in prostate cancer, with potential clinical implications in patient management. However, the ideal method with which to incorporate the information attained from the anatomic stage, histologic grade, PSA level, age, and comorbidity into a manageable prognostic score is uncertain and studies on this topic should be a high priority research objective.
The authors are grateful to the staff of the National Health Laboratory minister of health republic of Sudan for their cooperation during the study. They are also grateful to the Department of Histopathology and Cytopathology Faculty of the Medical Laboratory Sciences University of Sudan for help in processing and staining the specimens
Citation: Salih MM, Elsheikh LA (2019) Feulgen Reaction as a Marker for Identifying Prostatic Hyperplasia and Prostate Cancer. Andrology 8:201.
Received: 15-Apr-2019 Accepted: 06-May-2019 Published: 13-May-2019 , DOI: 10.35248/2167-0250.19.8.201
Copyright: © 2019 Salih MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.