Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Review Article - (2019)Volume 9, Issue 2
Extracellular vesicles (EV) are a heterogeneous group of membrane-enclosed structures that are shed from multiple cell types and can contain a variety of bioactive molecules including nucleic acids, lipids, and proteins. They range in size from 50 nm to 5,000 nm and, based on their size, are sub-classified into exosomes, microvesicles and apoptotic bodies. While the content and formation of EV are diverse, research has yet to pinpoint their physiological and pathological roles. This review presents an overview of EV, with focus on exosomes, the most studied EV, and on the cellular processes that mediate their biogenesis. Understanding these mechanisms can shed light on their roles in physiology and disease, and indicate their potential therapeutic efficacy in clinical settings, especially in musculoskeletal diseases.
Osteoarthritis related arthritides; Vesicles; Exosomes
Once exosomes are isolated from physiological fluids, it is essential to determine their purity. Exosomes contain certain universal lipids and proteins, including membrane transport proteins (RAB, GTPases), proteins used in MVB formation (Tsg 101 and Alix), heat shock proteins (hsc70), and tetraspanins (CD9, CD63, CD81, and CD82). Rab, SNARE and other membrane proteins are responsible for MVB docking and exosomal fusion with the plasma membrane [45]. These, in addition to the heat shock proteins and annexins, are integral to exosome intracellular assembly. Integrins, which mediate vesicle arget cell binding are also present on the surface of exosomes. Other proteins are specific for exosomes originated from specific cell types or tissues. In arthritis, specific biomarkers such as aggrecan, TGF-ß1 and LTBP-1 are used to identify exosomes secreted from disease-affected cells [43].
However, this method was effective thanks to the inherent capacity of curcumin to alter the fluidity of lipid membranes penetrate into the exosome lumen. Despite highly promising experimental results, there are still many challenges in the development of exosome-based therapies. One of the largest hurdles is the lack of large-scale production techniques. Typically, 1 ml of culture medium will yield<1 μg of exosomal protein, while effective doses of exosomes range from 10 ug to100 ug. Certain techniques have been found to enhance exosome production, including the application of stressors such as low pH, hypoxia and anti-cancer drugs. However, it is important to note that the effects of these techniques on exosome cargo are not fully understood, and caution must be taken in their evaluation. Another problem involves the selection of isolation methods. As discussed above, different isolation techniques exploit different properties of exosomes. Recent studies have indicated that exosomes preparations containing subpopulations of vesicles with different composition and size may have variable therapeutic potential depending on the isolation method. Future studies are warranted to overcome these technical hurdles and develop novel pharmacological therapies based on exosome-mediated delivery.
The dynamic nature of exosomes lends itself to many clinical applications, both for diagnosis and drug delivery. Since exosomal cargo mirrors the cell of origin’s macromolecules such as lipids, proteins, and nucleic acids, exosomes present a great potential as biomarkers for a variety of diseases, ranging from autoimmune disorders to cancer. In fact, databases have been created that correlate exosomal proteins and mRNAs with the corresponding diseases [104].
As exosomes are so heavily involved in intercellular communication via the plasma membrane, it is believed that target cells have greater tolerance for them than for inorganic drug vectors. In addition to biocompatibility, exosomes have the advantage of a long life in the blood circulation, the ability to target specific cells and little to no toxicity, which makes them an ideal target for future research in drug delivery. Exosomes carry MHC peptide complexes recognized by T lymphocytes without invoking an immune response from the host cells [105]. They are also able to traverse the blood-brain barrier and synovial membranes [106]. Many potent drugs are unable to traverse the blood-brain barrier and are rapidly cleared by phagocytes; conversely, appropriate tailoring of exosomes could afford improved drug delivery to the CNS. Due to their ability to transport miRNA, proteins and viral antigens, exosomes offer several avenues to deliver anti-inflammatory molecules throughout the body. Therefore, the use of exosomes as vehicles for targeted drug or gene delivery appears very promising.
The successful use of exosomes for drug delivery depends on the efficiency of the techniques used to load them. The techniques described in the literature include electroporation, incubation, and transfection. Electroporation consists of applying an electrical field to a suspension of exosomes and the drug/cargo of choice, forming pores in the exosome membrane that allow the drug to move into its interior. Transfection is the most commonly used method for loading therapeutic drugs into exosomes. Exosome donor cells are transfected to overexpress the gene product of interest, which the cell will abundantly package into the exosomes [107]. While a rare method, incubation has also been used as a technique to load exosomes. Curcumin was effectively loaded into exosomes after a 5-minute incubation period at 22°C [108,109].
Recent studies have highlighted the significance of exosomes in knee osteoarthritis (OA). OA pathology is derived from dysfunction in the balance between synthesis and breakdown of extracellular matrix (ECM), thought to benmediated by MMP-13 [87-89]. MMP-13 is produced by chondrocytes and fibroblast-like synoviocytes, and its expression is stimulated by IL-1ß and TNF- α [90,91]. Addition of IL-1ß treated chondrocyte EV to FLS increases MMP-13 production almost three-fold when compared to non-IL-1ß treated EV [92]. Conversely, addition of IL-1ß treated FLS EV to chondrocytes also increased MMP-13 production in addition to downregulating type II collagen [93]. Furthermore, recent studies have shown that miRNA content in exosomes is altered in OA synovial fluid relative to healthy controls and the changes are gender specific [94].
A pathological feature of OA includes the irregular hypercalcification of the tidemark, a layer of cartilage matrix found at the junction between articular cartilage and subarticular bone [95]. This is thought to lead to abnormal handling of mechanical stress at the joint, exacerbating degradation of articular cartilage [96]. Articular cartilage from the hips and knees of OA patients was shown to have more extracellular microvesicles than normal controls, and to exhibit high alkaline phosphatase activity [97]. Further studies have shown that microvesicles from OA chondrocytes contain annexin II, V and VI, all integral components of OA pathology [98].
Exosomes derived from OA synovium contain elevated levels of pro-inflammatory cytokines and chemokines, particularly IL-1ß [99]. Furthermore, exosomes from mesenchymal stem cells have been shown to promote both inflammatory and and antiinflammatory reactions via mTOR signaling and the control of mitochondrial functions [100,101]. Exosome-associated lncRNA KLF3-AS1 promotes chondrogenesis from progenitor cells [102]. Recent studies have also highlighted the role of exosomesderived from mesenchymal stem cells in promoting extracellular matrix synthesis and decreasing cartilage degeneration in animal models of OA [103].
The study of exosomes in psoriatic arthritis (PsA) is a very new and rapidly developing area of research. A recent study in China has begun studying exosomes as potential biomarkers for PsA. Twoexosome-associated long non-coding RNAs (lncRNA) - lnc- RP11-701H24.7 and lnc-RNU12 - were found to be increased in PsA patients compared to healthy controls, to be positively associated with clinical disease state [85]. Another recent study found five commonly expressed plasma exosomal microRNAs in patients with PsA, RA, gouty arthritis and psoriasis vulgaris, indicating similar inflammatory mechanism among these disorders [86].
Systemic Lupus Erythematosus (SLE) is characterized as dysfunction in the immune system leading to abnormal activation of immune cells, with production of autoantibodies that lead to tissue inflammation and damage [80]. Serum exosomes from SLE patients have been shown to induce high cytokine production in normal peripheral blood mononuclear cells. Mechanical disruption of the exosomes causes the inflammatory response to dissipate [81]. Additionally, SLE exosomes induce higher levels of IFN-α, TNF-α and IL-1ß when compared to healthy control exosomes with no difference in IL--6 production. Interestingly, the same study showed that exosomes from RA patients increase IL-6 but not IFN-α production, possibly because type 1 interferons are a key cytokine in SLE pathogenesis [81]. Further studies, aimed to elucidate the nature of the micro vesicles present in SLE patients, found unique vesicle populations containing annexin V and apoptosis-modified chromatin that activated bloodderived myeloid and plasmacytoid dendritic cells enhancing formation of neutrophil traps [82]. SLE TNF-α type patients were also found to have high levels of microvesicles from platelets, monocytes and T-lymphocytes [83]. In SLE patients the extracellular microparticle-associated chromatin could be a potential self-antigen. Extracellular chromatin is cleared by secreted deoxyribonuclease -1 like 3 (DNASE1L3) under normal conditions and DNASE1L3 deficiency could lead to loss of this tolerance mechanism and contribute to SLE [84].
Rheumatoid arthritis (RA) is a systemic autoimmune disorder characterized by production of auto antibodies, inflammation of synovium, and destruction of joint cartilage and bone [68]. EV are thought to play a major role in RA pathology. Higher levels of platelet-derived microvesicles have been detected in RA patients than healthy individuals, and their circulating levels correlated with disease severity [69]. Elevated microvesicles levels were found in inflamed joints [63,64]. Microvesicles released from granulocytes, monocytes and platelets have been found in the synovial fluid of RA patients [70] and shown to stimulate synoviocytes via IL-1ß, thus activating inflammation and promoting joint destruction. Synovial fluid microvesicles have been shown to induce coagulation [70], which may be contribute to cardiovascular comorbidities in RA patients. Exosomes derived from fibroblast-like synoviocytes (FLS) have been reported to contain citrullinated proteins and a membrane form of TNF alpha [62,71]. Osteoclast-derived exosomes containing microRNA have been reported to play an inhibitory role in osteoblast activity, exacerbating bone resorption [72] and paracrine signaling by vesicles in osteoblasts [73].
Interestingly, it has been shown that disease-modifying antirheumatic drug (DMARD) treatments decrease the relative amounts of plasma microvesicles from generated from platelets, endothelial cells, monocytes and leukocytes following 4 weeks of treatment with methotrexate and prednisone. Similar changes were noted in urine, indicating a correlation between disease activity and plasma levels of microvesicles [74]. However, other studies have reported no changes in microvesicle levels after 8 weeks of DMARD treatment, despite reduction in disease activity [75].
Current pharmacological treatments of RA mostly consist of medications that inhibit tumor necrosis factor-α (TNF-α) and multiple other targets including JAK kinase inhibitor, IL-6 receptor, CD20, CD80/86 co-stimulatory molecule and methotrexate [76]. Exosomes derived from immunosuppressive dendritic cells or body fluids of immunized organisms exert antiinflammatory and immunosuppressive effects in diseased tissues [77]; they could therefore offer a safer approach to direct the differentiation of naive immune cells towards an immunosuppressive phenotype and to activate the suppressor cells. Furthermore, immunosuppressive DC-derived exosomes and blood plasma- or serum-derived exosomes have shown potent therapeutic effects in animal models of inflammatory and autoimmune disease including RA. Engineered myelogenous leukemia cell line (K562 cells) expressing triple-costimulatory signals (CD80) with HLA-A2 as an antigen presenting cells promotes long-term propagation of CD8+ T cells, and the addition of other co-stimulatory molecules such as CD80 and CD83 support the expansion of naive T cell subsets [78]. Engineered Exosome- derived from engineered immune cells provide immunosuppression and offer several advantages. RA patients treated with exosomes isolated from autologous conditioned serum (ACS) containing anti-inflammatory cytokines have shown decreased joint pain and decreased levels of circulating inflammatory markers [73]. The use of exosomes derived from stem cells has great regenerative potential since microRNAs responsible for reduction of joint inflammation and damage can be incorporated into exosomes for therapy [79].
Exosomes are believed to be mainly involved in intercellular communication, a combination of paracrine and juxtracrine signaling through the exchange of proteins, lipids, mRNA, miRNA, and DNA between cells. The cargo carried by exosomes is specific for the cell type of origin. For example, exosomes derived from dendritic cells and B cells are involved in immunity, and their dissemination pathways can be exploited by retroviruses such as HIV, which possess the multi-vesicular body (MVB) machinery for propagation [52]. Exosomes derived from tumors contain oncogenes and onco-miRNAs that contribute to tumorigenesis, metastasis, and angiogenesis. Cancer cells release larger amounts of exosomes which allows fusion of exosome with other cells, contributing to the signaling mechanisms involved in tumorigenesis and metastasis [53]. Exosomes released by malignant breast cancer cells are taken up by other cells within the tumor, promoting its metastatic potential [54].
Exosomes not only serve as signaling vesicles; they also have significant effects on the microenvironment. Tumor-derived exosomes contain cytokines and growth factors that control cell functions in a variety of cells. Similarly, exosome-associated tetraspanins contribute to activating endothelial cells, and promote angiogenesis [55].
Certain exosomal proteins, such as the MET oncoprotein, mutant KRAS, and Tissue Factor, are directly linked with tumor cell proliferation and coagulation [22,56]. Prions and viruses can be transferred to host cells via exosomes in neurodegenerative diseases, including Alzheimer’s disease (AD). It has been proposed that retroviruses exploit exosomes both for the generation of viral particles and as a mode of infection. Based on this “Trojan exosome hypothesis” exosome uptake affords virus cells targeting by viruses through a mechanism independent of specific cell membrane receptor-viral envelop protein interaction [57].
Exosomes contribute to spreading pathological proteins (such as phosphorylated tau, or α-synuclein) across the nervous system in neurological conditions [58]. Exosome levels of total tau proteins (pT181-tau and pS396-tau) have been shown to be higher in AD patients than in case-controls, even 1–10 years before the diagnosis, suggesting that tau levels associated with blood exosomes can predict AD development before clinical onset [59]. In addition, the presence of exosome-associated tau phosphorylated at Thr-181 (AT270) in human cerebrospinal fluid suggests exosomes’ involevement in the pathogenesis of AD [60]. Exosomes also play an important role in immune surveillance through antigen presentation and the ability to modulate immune responses [19]. Exosomal long non-coding RNAs have also been implicated in aging and aging-related diseases, including atherosclerosis, Type 2 diabetes, osteoporosis, and osteoarthritis, by promoting cell senescence and apoptosis [61].
Exosomes contain autoantigens in patients with autoimmune diseases. Citrullinated proteins consisting of fibrinogen peptides and the CD5 precursor antigen are autoantigens involved in the pro-inflammatory response of rheumatoid arthritis (RA) [62]. Platelet-derived proinflammatory exosomes contain cytokines that induce synovial fibroblast secretion of IL-8, which causes joint inflammation and contributes to the irreversible process of cartilage erosion [1]. Long noncoding RNAs (lncRNA) such as Hotair are also found in the exosomes of RA patients. Hotair expression induces macrophage activation and matrix metalloproteinase (MMP) synthesis. Exosome-associated Hothair could therefore be used as a biomarker for rheumatoid arthritis [63]. Studies have also shown that IL-10 treated dendritic cells release exosomes that can prevent collagen-induced arthritis in rabbit and mouse models [64].
Due to their roles in intercellular communication, exosomes have been linked to viruses. Exosomes and viruses are similar in both size (under 300 nm in diameter) and composition. Similar to exosomes, viruses also contain proteins such as tetraspanins, cytoplasmic proteins, and heat shock proteins for proliferation. For these reasons, it can be very difficult to separate exosomes from the viruses themselves [65]. Exosomes can mediate both infection and immunity as they can transport viral content but also activate lymphocyte responses. In addition, exosomes transport to target cells antiviral response components, such as Toll-like receptor (TLR) ligands that alert neighboring cells to mount an immune response against the virus. Antigen presentation by exosomes induces immune cell activation. Inversely, exosomes can impair immune cell functions by transmitting viral content to target cells, facilitating virus binding to host cells, and inducing immune cell apoptosis [66]. Exosomes released from HIV-infected cells contain virusassociated proteins and genetic material that facilitate prolongation of the infection. By mediating delivery of virulent factors such as HIV Negative Regulatory Factor, exosomes can actually induce T cell apoptosis during the early stages of HIV infection [67]. Other viruses have also been shown to use exosomal pathways of infection. Epstein-Barr virus infects Bcells, causing release of EBV miRNA via exosomes. In addition, human intestinal epithelial cells infected with rotaviruses release exosomes containing various viral proteins as well as immunomodulators.
Due to their essential roles as transport vehicles for proteins, microRNA, and lipids, exosomes are being increasingly seen as important effectors of important physiological and pathological functions. Exosomes from different parts of the body have different functions - those derived from platelets are involved in the inflammatory response, while those secreted by antigenpresenting cells can carry MHC class I or II molecules, participating in immune responses [13].
Exosomes are associated with numerous diseases, ranging from cancer to arthritis. They can be isolated from a variety of biofluids, including saliva, urine, synovial tissue, plasma, and serum, which makes them convenient for diagnostic purposes lends great specificity and sensitivity for diagnostics. Exosomal cargo, sorted and highly concentrated by the ESCRT complex, consists of genetic material in the form of DNA, messenger RNA and miRNA, which can provide insight into the pathology of many different types of cancer. Several exosomal proteins serve as biomarkers, including tetraspanins and heat shock proteins for melanoma, epidermal growth factor (EGF) for bladder cancer, and Fetuin-A for kidney disease [46]. Exosomes transport misfolded proteins in patients with Parkinson’s disease [47], and exosomes with particularly high concentrations of tetraspanins, such as CD63, are possible indicators of melanoma [48] and other malignant tumors, as tetraspanin levels are elevated in variety of cancer cells [49].
Recent studies indicate that exosomes have a highly specific tropism for their cell of origin, which can be used to target them to diseased tissues and/or organs. For example, cancer cellderived exosomes have unique characteristics that can be of interest for drug transport system design [50]. Urinary exosomes could provide diagnostic information for liver and kidney conditions, as well as bladder and prostate cancer [51]. Because RNase present in biological fluids cannot degrade miRNAs contained in exosomes, the use of exosomal biomarkers provides a high level of stability for accurate and sensitive bio-diagnostics. Currently, alternative sources of biofluids, such as saliva and amniotic fluid, are being investigated along with blood and urine in order to devevop novel, more sensitive methods of diagnosis. Amniotic fluid, in particular, could provide fetal exosomal markers that can allow the early diagnosis of congenital conditions.
Multicellular organisms depend on intercellular communication to control a variety of cell functions and maintain homeostasis. While the mechanisms behind cell-cell contact and molecular signaling have been extensively studied, the importance of extracellular vesicles (EV) in intercellular communication has only recently been appreciated [1-3].
The first observation of EV is believed to have been made in 1946 by Chargaff and West, who wrote of procoagulant plateletderived particles found in normal plasma [4]. In the 1970s there followed an increasing number of independent EV observations, from the detection of vesicles in seminal plasma [5] to observations of tumor-originating membrane fragments [6]. The next large steps in EV research occurred in the 1980s, when studies of reticulocytes found that vesicles, in addition to being released from the cell surface, could also be released via fusion of multi-vesicular bodies (MVB) to the plasma membrane [7,8].
Lastly, numerous immunological separation methods can be used to isolate exosomes from biological fluids, including ELISA-based techniques or magnetic beads coated with specific antibodies. This method affords separating cell type-specific exosomes; however, it may alter EV’s biological activities and is not convenient to use with large sample volumes [43]. Nowadays, commercial reagents such as Exoquick (Takara Bio USA, Inc) are used to isolate exosomes from serum by a single overnight incubation, without the multiple long periods of differential centrifugation.
Once isolated from biological fluids, EV can be further analyzed by electron microscopy and various biochemical and biological approaches including genomics, proteomics and metabolomics. The characterization of the isolated vesicles is essential to ensure that the purified material contains the appropriate extracellular vesicle. The identification of biomarkers affords understanding exosome biological functions, and the characterization of their proteins, lipids and nucleic acids can provide indications of their tissue of origin. While there are universal proteins on all exosomes, the presence and quantitity of a variety of protein components depend on the specific cell the exosomes are secreted from [44]. Western blotting and ELISA can be used to identify these macromolecular components of EV.
Polymer-based precipitation consists of the addition of a large polymer (usually polythrilenglycol, or PEG) to a biological fluid with the resulting precipitation of large molecular complexes such as EV. However, the polymer may irreversibly denature EV components, affecting their biological activities and therefore hampering functional studies of the purified EV. Furthermore, the isolation is often incomplete, and results in EV with a low grade of purification [42].
Size-exclusion chromatography separates components of biological fluids based on their size. While this method is precise, and the structure of EV is not affected by shearing force, the process is time-consuming and quantitatively inefficient, which can limit the amount and biological activity of the purified material. Ultrafiltration membranes can also be used as a way to eliminate larger components from the exosomes; however, exosome adhesion to the filtration membrane results in reduced amounts of pure exosomes recovered by this method [42].
Differential centrifugation is the most common method for isolating EV [39]. By this method, the sample undergoes several centrifugation cycles to remove intact cells or debris. The supernatant is then processed through ultracentrifugation to obtain pure exosomes. While differential centrifugation is most commonly used, it is more effective with serum than plasma samples due to the higher viscosity of plasma. Important limitations of this technique include co-precipitation of apoptotic bodies and various nucleosomal fragments, as well as the need for low viscous biofluids. Longer centrifugation times and higher centrifugal force are needed to compensate for both limitations [40]. Sucrose density gradient centrifugation separates vesicles based on differences in flotation densities [41]. Density gradient centrifugation is best used for low-density exosome purification but is very sensitive to alterations in centrifugation time [19].
A number of techniques have been developed to isolate and analyze EV taking advantage of their structural and biochemical features. These techniques include differential or density gradient centrifugation, chromatography, filtration, polymerbased precipitation, and immunological separation.
Extracellular vesicles (EV) are membrane-contained vesicles whose release from cells has been conserved through evolution, from prokaryotes to unicellular and multicellular eukaryotes [17]. Heterogenous in structure, EV carry a wide variety of molecules, ranging from proteins to multiple RNA species, DNA, and lipids [18-21]. EV release often occurs into bodily fluids such as serum, plasma, and urine. To elicit their functional effects, EV bind to the plasma membrane of target cells, inducing phenotypic changes in the cell via cell surface signal initiation or vesicle internalization [22]. Extracellular vesicles are categorized into three main categories-microvesicles, apoptotic bodies, and exosomes, based on intracellular origin and size.
Microvesicles (also termed microparticles, particles or ectosomes in the literature) are a heterogeneous population of vesicles primarily formed through the direct outward budding and fission of the plasma membrane, followed by release into the extracellular space [23]. Microvesicles range in size from 100 nm to 1,000 nm [3]. The blebbing of the plasma membrane is accompanied by localized shifts in plasma membrane components, with a resulting local enrichment of microvesicle cargo components as well as changes in membrane curvature and rigidity [24]. Microvesicle budding utilizes the ARF6 (ADPribosylation factor 6, a RAS-related GTPase) and the ESCRT (endosomal sorting complexes required for transport) system [25]. Studies have identified ARF6 as integral to the regulation of selective protein recruitment into microvesicles, and of microvesicle shedding through its function in cell invasion, peripheral actin remodeling, and endocytic trafficking [26,27]. Unlike other sub-classes of EV, microvesicles can vary greatly in composition and morphology depending on cell type (s) and health or disease conditions [28]. They contribute to various physiological and pathological processes, including tumor invasion and metastasis, and are believed to play a role in inflammation, coagulation, stem-cell renewal and expansion, evasion from the immune response, and bone mineralization [29]. For example, microvesicles secreted by skeletal cells have been found to play a role in initiating bone mineralization, while microvesicles secreted from endothelial cells or adipocytes have been implicated in angiogenesis and glucose metabolism, respectively [30-32].
Apoptotic bodies, or apoptosomes, are extracellular structures released from cells undergoing apoptosis (programmed cell death). A cell undergoing apoptosis goes through multiple stages, starting with fragmentation of nuclear chromatin and progressing to membrane blebbing, with formation of membrane-enclosed vesicles containing disintegrated cell components [33]. After release, apoptotic bodies are phagocytosed by local macrophages through interactions between the vesicle cell membrane and the phagocytic receptors [34]. While apoptotic bodies range in size from 500 to 4,000 nm, oftentimes during apoptosis vesicles in the 50-500 nm range are also released [33-35]. It is currently unclear whether they are formed through the same processes as the larger vesicles released. It has been suggested that uptake of apoptotic bodies can result in transfer of genetic material from a dying cell to a viable cell. For example, apoptotic bodies from human c-myctransfected cells confer loss of contact inhibition on murine fibroblasts in vitro [36].
Unlike apoptotic bodies and microvesicles, exosomes originate in the endosomal system, and are typically smaller than 100 nm [3]. Early endosomes mature into late endosomes, or MVB. As this process occurs, the ESCRT machinery mediates the invagination of the endosomal membranes to form intraluminal vesicles (ILV), which are released as exosomes when the MVB fuse with the plasma membrane [37]. Exosomes have a multitude of functions involved in cell-cell communication and transport, depending on the cell type from which they are secreted. They can transfer proteins, lipids, and nucleic acids into, and alter gene expression in target cells. Like the plasma membrane they originate from, exosomes consist of a lipid bilayer with higher cholesterol and phosphatidylserine levels, which may facilitate internalization through endocytosis, phagocytosis, and fusion [38]. Exosomes are the most studied among EV. We will therefore focus our review on this type of EV.
In the 1990s, studies began showing an increasingly clear connection between the release of EV and the immune response, such as the capacity of EV derived from Epstein-Barr virus-transformed B to induce a T-cell response [9] Multiple studies have shown that EV contain multiple RNA species, including microRNAs (miRNA), making them a potentially important player in cell-cell communication [10-15]. The number of publications on EV has skyrocketed in the past 10 years (Figure 1), and the increased interest has led to a deeper characterization of the various types of EV, as well as the standardization of the nomenclature, also thanks to the creation of the International Society of Extracellular Vesicles (ISEV) and the NIH program, Extracellular RNA Communication Consortium (ERCC) [16].
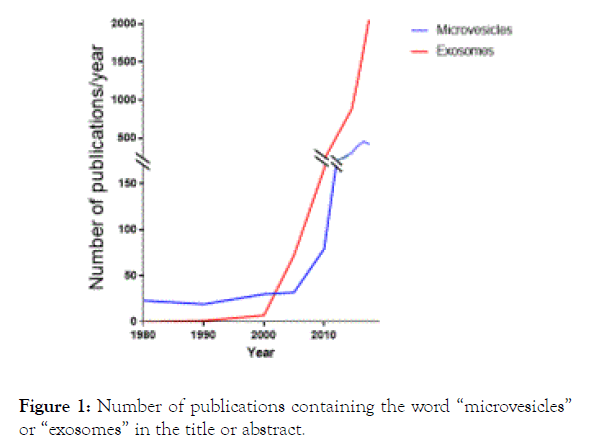
Figure 1. Number of publications containing the word “microvesicles” or “exosomes” in the title or abstract.
Citation: Attur M, Mignatti P, Han T, Attur MG (2019) Extracellular Vesicles Biology and its Emerging Role in Osteoarthritis and Related Arthritides. Rheumatology (Sunnyvale). 9:254. DOI: 10.35248/2161-1149.19.9.254
Received: 08-Oct-2019 Accepted: 18-Oct-2019 Published: 23-Oct-2019 , DOI: 10.35248/2161-1149.19.9.254
Copyright: © 2019 Attur M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.