Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2022)Volume 11, Issue 4
Xylopia aethiopica is normally used as a major spice in pepper soup for post parturient women. Their spouses are advised to eat the nutrient rich pepper soup also.
Aim: The aim of this study is to investigate how this spice affects the hormone profile of male and female vertebrates using albino rats. To do this, aqueous extraction of two varieties of Xylopia and assessment of the compounds that comprise the two varieties was done. In vitro experiments to ascertain the effect of Xylopia on isolated rat uteri was considered necessary. Also, haematology and histopathology of the testis was also considered important. The two varieties of Xylopia used; Xylopia Ripe Fruit Extract (XRFE) and Xylopia Unripe Fruit Extract (XUFE) extracts were applied to strands of rats isolated uterine smooth muscle preparation in De Jalon solution and bubbled with air. In vivo; thirty rats of fourteen weeks of age were used. Fifteen males were divided into three groups of five each. Group A was control given 2 ml/kg of distilled water while groups B and C were given XRFE and XUFE at a dose of 800 mg/kg for thirty days. The fifteen females were divided into three as in males and treated accordingly. After thirty days, the rats in all the groups were sacrificed using mild ether for sedation and blood collected through cardiac puncture for haematology in heparinized bottles and serology in test tubes.
Result: In vitro experimentations show that Xylopia relaxes the uterus in dose dependent manner. Hormonal profiling of the sera from in vivo experimentation in males revealed that Xylopia treatment reduces testosterone levels. The rate of testosterone reduction is more expressed in XRFE (P ≤ 0.01) treated than in XUFE (P ≤ 0.05) treated males. At the other hand, oestrogen secretion is significantly (P ≤ 0.01) enhanced in the females in XRFE treated females and significantly (P ≤ 0.05) reduced in XUFE treated females.
Conclusion: Xylopia treatment in the females lead to induced secretion of oestrogen which suppresses the secretion of mono amine oxidase thereby averting Post Parturient Depression (PPD) while in the males Xylopia treatment is postulated to be a handy male contraceptive. However more work should be done to confirm whether Xylopia treated males becomes temporarily infertile or not.
Xylopia ; Testosterone; Oestrogen; PPD; Male contraceptive
A hormone is any substance that acts at the cellular level to initiate, stop or modulate a cellular process. The site of action can be nearby or at a distant target. Hormones can act through paracrine, autocrine or intracrine mechanisms [1].
Xylopia aethiopica synonyms: Negro pepper, African pepper, guinea pepper and spice tree, is an ever green aromatic tree growing up to 15 m-30 m high and about 60–70 cm in diameter [2]. It is native to the lowland rainforest and moist fringe forest in the savanna zones of Africa, but largely found in West, Central and Southern Africa. An attractive spicy flavor is obtained after Negro pepper is smoked during the drying process [3]. Xylopia aethiopica leaves are simple, alternate, oblong and elliptic to ovate. Its flowers are bisexual, solitary or in 3-5 flowered fasicles or in strange, sinuous, branched spikes, or cymes, up to 5.5 by 0.4 cm and creamy green [4].
Fruits of Xylopia aethiopica look like small, twisted bean pods which are dark brown, cylindrical, 2.5 to 5 cm long and 4 to 6 mm thick. Each pod houses about 5 to 8 kidney shaped seed grains of approximately 5 mm length [5].
Plant material: The fruits were purchased at Ubani market in Umuahia North local government area of Abia state and were authenticated at the department of forestry, college of natural and environmental management, Michael Okpara university of agriculture, Umudike. The picture below shows Xylopia ripe fruits (Plate A) and Xylopia unripe fruits with seed (Plate B) (Figure 1).

Figure 1: The picture shows Xylopia ripe fruits (Plate A) and Xylopia unripe fruits with seed (Plate B).
Equipment: Weighing balance, gastric gavage, measuring cylinder, oven, milling machine, water bath, drinkers, feeders, aluminum cages and physiograph (PC, 2004 model)
Consumables: Vital feed (finisher), water, distilled water, cotton wool, syringes and needles and De Jalon solution.
Animals: 70 albino rats were used; 30 rats both male and female picked at random for toxicity testing, 5 females per sample of xylopia extract for in vitro experimentation and 30 rats for in vivo experimentation.
All the experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) FV-UIACUC- 2020-0262 of University of Nigeria, Nsukka and the procedure approved were in compliance with guide for the care and use of laboratory animals, eighth edition which upholds recognition and alleviation of pain in laboratory animals according to the institute for laboratory animal research publication.
Methods
Preparation of extract: The plant was sundried and pulverized with the aid of milling machine. 1000 gram of the weighed plant ripe fruit powder (XRFE) was soaked in 3000 mls of water and boiled for 30 minutes and filtered using mounilex sieving cloth. The unripe fruit with seed powder (XUFE) was also weighed and exact quantity as in ripe fruit (1000 g) was soaked in 3000 mls of water and boiled for 30 minutes and filtered using mounilex sieving cloth as in ripe fruit. The extracts were further dried in an electric oven to obtain a light brown paste for the ripe fruit (XRFE) and dark brown paste for the unripe with seed variety (XUFE) of Xylopia aethiopica and stored in a freezer until needed. When needed, 1 gm of the aqueous extracts was dissolved in 5 ml of distilled water giving a concentration of 200 mg/ml for use each day of the experiment.
Gas Chromatography-Mass Spectrometry (GCMS): The characterization of the phytochemicals in two varieties of Xylopia was done using Masshunter, Agilent Technologies, 5977, US1447 L431, 6.00.21. Identification of the active phytochemicals in the extract was done by comparison of their retention indices, peak area and mass spectra fragmentation pattern with 250 compounds stored in the data base of Agilent technologies, Inc.
Toxicity testing: Thirty rats grouped into six of five per group were given graded doses of the Xylopia at 500 mg per kg body weight, 1000, 2000, 3000, 4000 and 5000 mg/kg respectively and monitored for any sign of toxicity which include: hyperactivity, abnormal gait, spasms in rear legs and hyperreactivity. When no sign of toxicity was observed, the rats were further left for seven days.
In vitro experiments: These were carried out in the physiology laboratory of the department of physiology and pharmacology in Michael Okpara University of Agriculture, Umudike (MOUAU).
De jalon solution; the physiological salt solution which is most suitable for isolated rat uterine preparation was prepared such that each liter of water contained, Nacl: 9 g, kcl; 0.42 g, Cacl; 0.6, NaHCO; 0.50. Glucose; 0.50 [10].
The female rats used were sedated using mild ether soaked in cotton wool and cervical dislocation carried out to avoid pain. The lower abdomen was opened (evisceration). The two horns of the uterus were carefully traced, isolated, trimmed off fatty tissue attachments and then transferred into a beaker containing De jalon solution at thirty seven degree centigrade and aerated. The horns, one after the other was mounted in a 35 ml organ bath containing De jalon solution at thirty seven degrees centigrade and fully aerated. The mounted tissue was allowed to equilibrate for 30 minutes, after which dose relationship were established using: Ripped (XRFE) and unripe (XUFE) of Xylopia aethiopica, oxytocin and salbutamol.
In vivo experiments: Adult albino rats of fourteen weeks of age were obtained from veterinary physiology and pharmacology department, (M.O.U.A.U.) Abia state, Nigeria. The experimental animals were acclimatized for two weeks in clean aluminum cages in which they were housed at room temperature. The animal were fed ad libitum with vital feed and supplied with clean drinking water all through the study. The treatments were done through the oral rout of administration and the study lasted for 30 days. The total number (30) of experimental animals used for this study was divided into two groups of 15 each, which comprised of the male and female group. The male and female animals were randomly assigned to three experimental group of five rats each (Groups: A, B, and C). Group A which served as control was given 2 ml/kg body weight of distilled water, group B was treated with 800 mg/kg of XRFE and group C was treated with 800 mg/kg of XUFE. All the treatments were done through the oral rout of administration. At the end of treatments, the animals were sedated with mild ether and cervical dislocation done to alleviate pain as previously expressed. Exsanguination and evisceration were carried out. Two different blood samples were collected through cardiac puncture from each animal; the first sample was collected with heparinized sample bottle for hematological assessment. Red Blood Cells (RBC), Hemoglobin Concentration (Hb), packed cell volume (PCV), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin Concentration (MCHC), Mean Corpuscular Hemoglobin (MCH) and total White Blood Cell (WBC) were determined using standard methods, and the second sample was collected with test tubes and kept slanting for 24 and the sera separated for hormone profiling. The ones that did not separate were centrifuged at 2500 rotations and the sera collected using Pasteur pipette into sterile sample bottles. The samples were sent to Federal Medical Center Umuahia, Abia State, Nigeria and Estrogen, Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) were profiles for the females while Testosterone was profiled for the males using enzyme immune absorbent assay through kits. Thereafter, a graph for known quantities of these hormones was used to extrapolate the levels of the individual hormones found in each rat.
Histopathology
Testes were fixed in Bouin’s fluid for 48 hours after evisceration and testes separation. Thereafter, the organs were fixed in 70% alcohol and cleared with Xylene. The tissues were embedded in paraffin wax, sectioned with microtome, stained with Haematoxylin and Eosin (H and E), covered with cover slips and mounted in a Canada balsam. Examination of slides was under light microscope (X40, X100, X400 and X1000) magnifications. Photomicrographs were taken using digital microscope DN-10, DC 7.5 V.
Statistical analysis: Results were expressed as mean ± standard error of mean (SEM) and percentages. Students T-test at 95% and 99% confidence interval level of significance were used to access significant differences where necessary.
Gas Chromatography-Mass Spectrometry (GC-MS) analysis result revealed that: Xylopia Ripe Fruit Extract (XRFE) contains among other things; L-aspartic acid and eucalyptol while Xylopia unripe fruit extract contains among other things Laspartic acid and morpholine.
Toxicity evaluation of Xylopia aethiopica revealed that the substance is safe even at a dose of 5,000 milligram per kilogram body weight (Table 1).
| Group | Dose administered (mg/kg) | Number of deaths | Percentage mortality (%) |
|---|---|---|---|
| 1 | 500 | 0/5 | 0.00 |
| 2 | 1000 | 0/5 | 0.00 |
| 3 | 2000 | 0/5 | 0.00 |
| 4 | 3000 | 0/5 | 0.00 |
| 5 | 4000 | 0/5 | 0.00 |
| 6 | 5000 | 0/5 | 0.00 |
Table 1: Toxicity evaluation of Xylopia aethiopica (XRFE and XUFE).
In vitro results of Xylopia aethiopica treated animals
The application of XRFE and XUFE caused inhibitory effect on the isolated uterine tissue when compared to basal contractions.
The relaxations induced by XRFE and XUFE were dose dependent (Tables 2 and 3). For instance, with a basal contraction of 3.25 ± 0.25, 333.33 μg/ml of XRFE extract produced an amplitude inhibition of 0.63 ± 0.13 and a percentage inhibition of 80.20 ± 4.61. While 1333.33 μg/ml of XRFE extract with a basal contraction of 3.50 ± 0.29 produced an amplitude inhibition of 0.13 ± 0.13 and a highest percentage inhibition of 96.88 ± 3.13 (Figures 2 and 3).
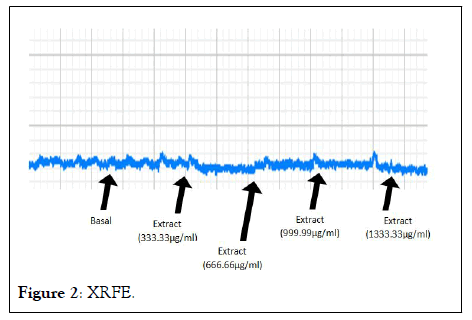
Figure 2: XRFE.
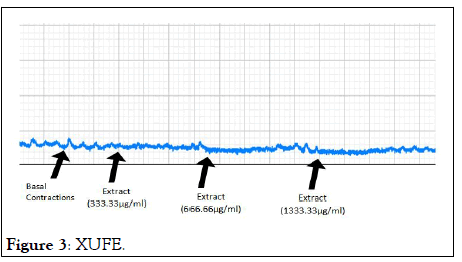
Figure 3: XUFE.
| Groups | FBC (µg/ml) | Basal | Amplitude of response (mm) | % Activity (inhibition) |
|---|---|---|---|---|
| 1 | 333.33 | 3.25 ± 0.25 | 0.63 ± 0.13 | 80.20 ± 4.61 |
| 2 | 666.66 | 3.25 ± 0.25 | 0.38 ± 0.13 | 87.48 ± 4.18 |
| 3 | 1333.33 | 3.50 ± 0.29 | 0.13 ± 0.13 | 96.88 ± 3.13 |
Table 2: In vitro Xylopia aethiopica Ripe Fruit Extract (XRFE).
| Groups | FBC (µg/ml) | Basal activation | Amplitude of response (mm) | % Activity (inhibition) |
|---|---|---|---|---|
| 1 | 333.33 | 4.75 ± 0.25 | 2.50 ± 0.29 | 47.50 ± 4.79 |
| 2 | 666.66 | 4.75 ± 0.25 | 0.75 ± 0.14 | 83.75 ± 3.75 |
| 3 | 1333.33 | 5.75 ± 0.25 | 0.13 ± 0.03 | 93.75 ± 6.25 |
Table 3: In vitro Xylopia aethiopica Unripe Fruit Extract (XUFE).
Hormonal profile
Hormonal profiling of male and female albino rats treated for 30 days revealed that XRFE significantly reduced testosterone level from 8.20 ± 0.15 in control to 1.31 ± 0.12 at 99% confidence interval while XUFE significantly reduced testosterone level from 8.20 ± 0.15 in control to 5.39 ± 0.13 at 95 confidence interval. At the other hand, XRFE significantly increased estrogen level in treated females from 147.36 ± 1.62 in control to 179.40 ± 2.46 while XUFE significantly (P ≤ 0.05) reduced estradiol level from 147.35 ± 1.62 to 123.22 ± 1.26 (Table 4, Figures 4 and 5).
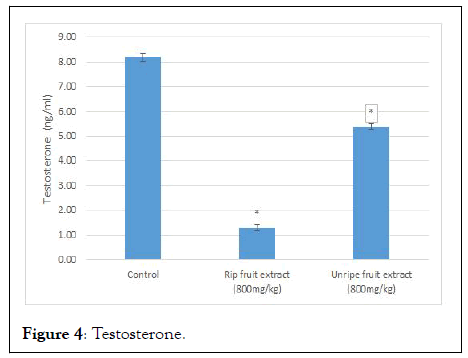
Figure 4: Testosterone.
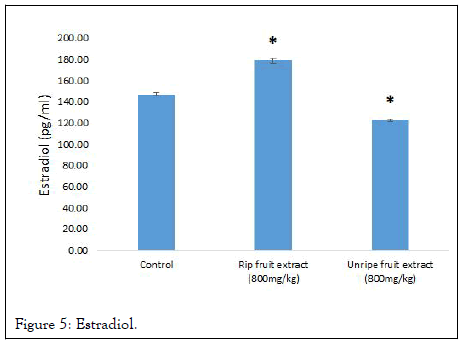
Figure 5: Estradiol.
| Groups | Treatment | Male (Mean) | Female (Mean) | ||
|---|---|---|---|---|---|
| Testosterone (ng/ml) | FSH (miu/ml) | LH (miu/ml) | Estradiol (pg/ml) | ||
| 1 | Control | 8.20 ± 0.15 | 4.97 ± 0.02 | 5.00 ± 0.05 | 147.36 ± 1.62 |
| 2 | XRFE (800 mg/kg) | 1.31 ± 0.12* | 3.43 ± 0.05* | 3.56 ± 0.10* | 179.40 ± 2.46* |
| 3 | XUFE (800 mg/kg) | 5.39 ± 0.13* | 3.04 ± 0.08* | 3.49 ± 0.02* | 123.22 ± 1.26* |
The superscripts indicate significant difference in a column.
Table 4: Hormonal profiling of male and female rats treated with XRFE and XUFE.
Hematology
In the male albino rats treated for 30 days, both XRFE and XUFE significantly (P ≤ 0.05) reduced RBC, PCV and Hemoglobin (Hb) when compared with control but significantly (P ≤ 0.05) increased WBC from 9.54 ± 0.38 in control to 10.22 ± 0.30 in XRFE. There was no significant difference in platelets number between XRFE treated males and control. However, there was significant (P ≤ 0.05) reduction in platelets in XUFE treated males when compare to control from 32.80 ± 1.98 in control to 25.60 ± 0.58. However, there was no anemia observed in the treated rats since the value of Mean Corpuscular Volume (MCV) remained within range 70.76 ± 0.67 in control, 71.90 ± 1.28 in XRFE treated males and 72.58 ± 0.47 in XUFE treated males and all the values are below 96 fl (Table 5).
| S/No | Blood parameters | XRFE (800mg/kg) | XUFE (800mg/kg) | Control |
|---|---|---|---|---|
| 1 | Red blood cells (x 1012/L) | 5.76 ± 0.16* | 6.08 ± 0.10* | 6.63 ± 0.16 |
| 2 | Packed cell volume (%) | 35.70 ± 5.47* | 44.08 ± 0.47 | 46.44 ± 0.81 |
| 3 | Haemoglobin (g/dL) | 12.10 ± 0.18* | 12.76 ± 0.10* | 13.88 ± 0.24 |
| 4 | White blood cells (x 109/L) | 10.22 ± 0.30⁎ | 11.04 ± 0.58* | 9.54 ± 0.38 |
| 5 | Platelets (x 109/L) | 32.60 ± 2.48 | 25.60 ± 1.12* | 32.80 ± 1.98 |
| 6 | Mean corpuscular volume (fL) | 71.90 ± 1.28 | 72.58 ± 0.47 | 70.76 ± 0.67 |
| 7 | Mean corpuscular hemoglobin (pg) | 21.07 ± 0.54 | 20.99 ± 0.31 | 21.20 ± 0.67 |
| 8 | Mean corpuscular hemoglobin conc. (g/L) | 29.45 ± 0.17 | 28.97 ± 0.29 | 29.95 ± 0.83 |
The superscript indicates significant difference in a row.
Table 5: Hematology of male rats treated with XRFE and XUFE.
In the female rats treated also for 30 days, there was significant (P ≤ 0.05) reduction in RBC, PCV and Hb in both XRFE and XUFE treated female rats. However, just as in male, there was increase in in WBC from 9.92 ± 0.42 in control to 11.84 ± 0.60 in XRFE treated females and 12.70 ± 0.28 in XUFE treated females. Unlike the males, there was no significant difference among platelets in control and Xylopia treated females (both ripe and unripe). Furthermore, there was no anemia observe since just as in males, the value of MCV remained within range (Table 6).
| S/No | Blood parameters | XRFE (800 mg/kg) | XUFE (800 mg/kg) | Control |
|---|---|---|---|---|
| 1 | RBC (x 1012/L) | 5.46 ± 0.10* | 5.10 ± 0.07* | 6.16 ± 0.14 |
| 2 | PCV (%) | 41.12 ± 0.85* | 38.66 ± 0.73* | 46.26 ± 0.63 |
| 3 | Hemoglobin (g/dL) | 12.54 ± 0.14* | 11.58 ± 0.17* | 13.22 ± 0.10 |
| 4 | WBC (x 109/L) | 11.84 ± 0.60* | 12.70 ± 0.28* | 9.92 ± 0.42 |
| 5 | Platelets (x 109/L) | 33.20 ± 1.46 | 30.80 ± 1.16 | 34.40 ± 1.69 |
| 6 | MCV (fL) | 75.20 ± 0.23 | 75.80 ± 0.62 | 75.22±1.55 |
| 7 | MCH (pg) | 22.82 ± 0.60 | 22.71 ± 0.11 | 21.51 ± 0.42 |
| 8 | MCHC (g/L) | 30.50 ± 0.67 | 30.00 ± 0.19 | 28.60 ± 0.21 |
The superscripts indicate significant difference in a row.
Table 6: Hematology of female rats treated with XRFE and XUFE.
Histopathology: The image of the testes at 1000 resolution showed significant halting of spermatogenic activity after spermatogonia stage in XRFE treated male rats while spermatogenic activity halted at secondary spermatocyte stage in XUFE treated rats. The control treated with distilled water showed full spermatogenic activity from spermatogonia, primary spermatocytes, secondary spermatocytes, round spermatids and finally elongated spermatids (Figures 6-8).
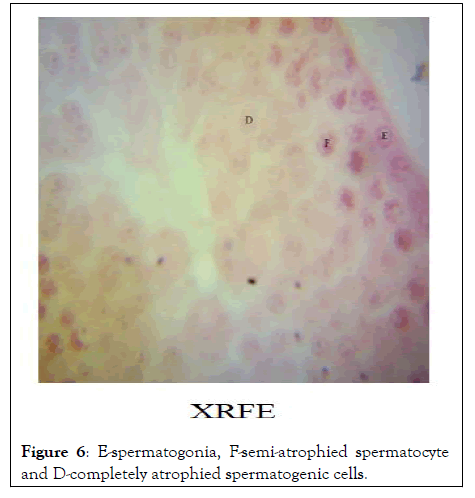
Figure 6: E-spermatogonia, F-semi-atrophied spermatocyte and D-completely atrophied spermatogenic cells.
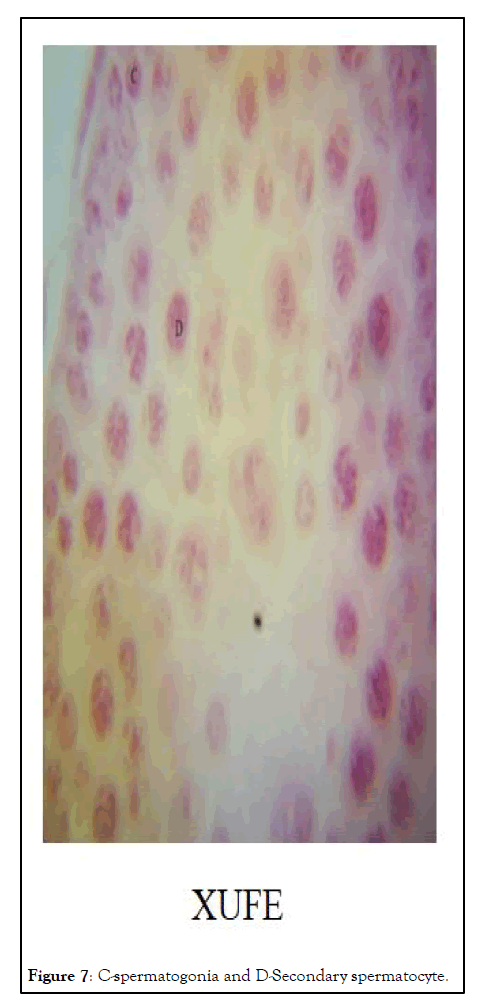
Figure 7: C-spermatogonia and D-Secondary spermatocyte.
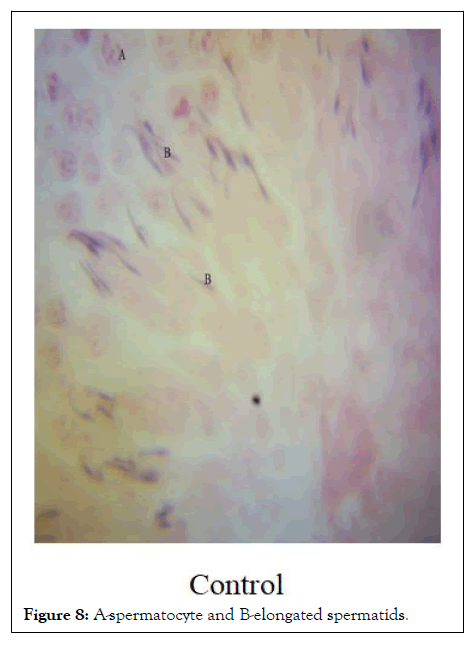
Figure 8: A-spermatocyte and B-elongated spermatids.
Gas Chromatography-Mass Spectrometry (GCMS) analysis of Xylopia aethiopica revealed that both Xylopia Ripe Fruit Extract (XRFE) contain L-aspartic acid which is a building block for protein in the body. However XRFE contains eucalyptol which is a chemical used to cleanse the respiratory tract. This agrees with Ahamefula, et al. [2]. Who said that the extracts of the fruit are used in the treatment of cough, biliousness, bronchitis, rheumatism, dysentery, malaria, uterine fibroid and amenorrhea? For post parturient female, eucalyptol is useful since it enables the female to suckle the neonates without interruptions of coughing.
The result of toxicity testing shows that Xylopia is tolerable even at very high dose of 5,000 mg/kg body weight. This shows that Xylopia aethiopica is safe for use as medication.
The hormonal profiling of male rats treated with Xylopia aethiopica at a dose of 800 mg/kg revealed that XRFE significantly reduced serum testosterone level (P< 0.01). However, XRFE did not reduce the number of seminiferous tubules found in the testis instead, the meiosis activity of the spermatocytes were suspended. Subsequently, other cells involved in spermatogenesis were atrophied. These show that treatment with Xylopia Ripe Fruit Extract (XRFE) can be a strong male contraceptive with 99% confidence interval since the treatment affects the middle and the later stages of spermatogenesis this agrees with Ologhaguo, et al. [9] who suggested that Xylopia aethiopica possesses antifertility potentials which could be explored for male contraceptive. Interestingly, Xylopia Unripe fruit extract (XUFE) at same dose of 800 mg/kg also significantly reduced serum testosterone level in the male at 95% confidence interval and affects only the later stage of spermatogenesis because the secondary spermatocytes could be viewed. This revealed that both varieties of Xylopia could be used for male contraceptive but more work should be done to ascertain the time it takes for treated rats to resume spermatogenesis post treatment. Is there variation in the time interval between XRFE and XUFE treated animals? The result will assist one to conclude on the variety of choice for male contraception [11,12].
In the females, XRFE treatment led to increase in serum estradiol level in post parturient female which is advantageous in two ways:
Xylopia soup given as first meal postpartum which leads to increase in synthesis of estrogen may alleviate postpartum depression. Postpartum Depression (PPD) occurs as a result of excessive drop in estrogen and progesterone level postpartum leading to increase in mono amine oxidase which causes depression. This agrees with Dowlati, et al. PNAS, who wrote that selective dietary supplementation in early postpartum, is associated with resilience against depressed mood.
Secondly, the increased estrogen synthesized as a result of Xylopia meal is possible to negatively feedback the hypothalamus to stop the secretion of Gonadotropin Releasing Hormone (GnRH) leading to halting of the secretion of Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) from the pituitary. Therefore the female has enough time to recuperate from pregnancy and parturition events before resuming reproductive cycling.
The hematology of male rats treated with Xylopia aethiopica revealed that the total white Blood Cells (WBC) significantly (P ≤ 0.01) increased in XUFE treated rats. This shows that the body’s immune static mechanism may be activated for increased functioning with the treatment.
Xylopia aethiopica treatment is found to be useful postpartum in females in order to avoid postpartum depression due to significantly increased estrogen levels in XRFE treated females. Also, XUFE contains morpholine which is a strong pain killer. It is suggested that combining both XRFE and XUFE may be a better therapeutic regimen for women post-partum. This is because parturition events are normally painful during and after delivery and pain reduction is highly recommended especially through consumption of organic substances.
In the male, XRFE reduced serum testosterone. Therefore, it is suggested that XRFE be the drug of choice for male contraceptive. However, more work should be done to ascertain whether XUFE is a strong contraceptive. If it is, then XUFE should be preferred to XRFE since the Histopathologically lesions shows that XUFE affects only the later stage of spermatogenesis and reversal back to normal will be quicker when compared to XRFE in which the primary and secondary spermatocytes as well as the spermatids were all atrophied due to the treatment which is a strong indication of halting of spermatogenesis leading to infertility. However, more work should be done to ascertain the length of time it takes for treated males to restore spermatogenesis post treatment.
We are grateful to our institution; Michael Okpara University of Agriculture, Umudike for giving us freedom to research with our scarce resources. We are thankful for being allowed to use the physiograph in department of veterinary physiology and pharmacology in the college of veterinary medicine. We are also grateful to Mr. Agbakwuru in the department of veterinary anatomy in the same college for assisting in the preparation of the slides for histopathology.
Ethic approval: All the experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC); FV-U-IACUC-2020-0262 of University of Nigeria, Nsukka Consent of Publication: All the authors unanimously consent to this publication.
Competing interests: There are no competing interests.
There was no internal or external funding received for this research.
First author (Nwankudu, O. N.) Designed the research, supervised the research, viewed and interpreted the slides, proof read the work and wrote up the paper for publication and is the corresponding author. Second author (Chibundu Amarachi) did the experimentation and the write up and did the statistical analysis.
Data are available upon request from the first author.
Citation: Nwankudu ON, Chibundu AR (2022) Evaluation of the Effect of Xylopia aethiopica on the Hormonal Profile of Vertebrates. Andrology. 11:263.
Received: 13-Aug-2022, Manuscript No. ANO-22-17902; Editor assigned: 16-Aug-2022, Pre QC No. ANO-22-17902(PQ); Reviewed: 01-Sep-2022, QC No. ANO-22-17902; Revised: 09-Sep-2022, Manuscript No. ANO-22-17902(R); Published: 16-Sep-2022 , DOI: 10.35248/2167-0250.22.11.263
Copyright: © 2022 Nwankudu ON, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.