Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2013) Volume 2, Issue 1
Background: The contributions of host factors, such as age, in male infertility are still poorly understood. Thus, the present work has aimed to evaluate the effect of patient’s age on semen parameters of infertile males.
Method: Semen samples from 52 infertile patients aged 21-52 years (mean 30.8 ± 6.7) were analysed by conventional semen analysis methods, flowcytometry analysis for sperm DNA integrity, and colorimetric assay for total antioxidant capacity.
Results: The study revealed that, as patient’s age increases, there was a significant reductionin sperm density, motility, percentage of morphologically normal spermatozoa, total antioxidant capacity and DNA integrity.
Conclusion: The study results demonstrated the negative influence of increasing patient’s age on semen parameters; and the possible role of patient’s age in the aetiology of male infertility.
<Keywords: Antioxidant capacity; Infertile male; Semen analysis;Sperm DNA
Infertility is a growing medical and social problem in the world; and approximately one of five couples has problems with conception [1]. Several fertility workers did focus in their search on female etiological factors for infertility, despite the fact that assessment of male fertility potential is of great importance. Male partner may involve in the aetiology of half of cases, either as primary or secondary causes [2]. Still unsatisfactory diagnostic strategy for male infertility causes is applied in many clinical centres in the world [3].
Many recognized diseases can disturb, or even permanently damage male fertility; some are congenital others are acquired. However, in several cases the exact cause is still unknown (idiopathic) [4]. In the last few decades, there was growing concern about the possible threat of the environmental chemical and physical agents to male fertility. Factors such as genital infection, smoking, and radiotherapy are frequently disturb the biochemical events that occur during spermatogenesis [5]. Furthermore understanding the effect of male age on fertility has become increasingly important in public health because a growing number of men are choosing to father children at older ages [6].
It is clearly recognized that, women develop reproductive quiescence around 50 years of age [7-9]. In contrast in men, aging follows a more gradual time course with functional disturbance at several sites within the HPG axis, including central neuroendocrine regulators, hypothalamic gonadotropin-releasing hormone, pituitary gland gonadotropins, and testicular testosterone [9-11] . Still, men commonly do not experience complete reproductive senescence and maintain spermatogenesis well into old age [7-9,12].
Semen quality is usually considered to be a proxy measure of male fertility, and changes in semen quality can occur after exposure to toxic agents, or from host factor effects such as age [6,13]. Semen analysis provides limited prognostic information on male fertility and recent studies indicated that the integrity of sperm DNA may be a more accurate predictor of fertility [13]. Another important fertility parameter is Oxidative stress (OA); it is a product of the inequity between reactive oxygen species (ROS) and antioxidants in the body. It is a potent mechanism that can lead to sperm damage and male infertility [14]. Normally, the seminal plasma contains specialized antioxidant system that provides effective protection against (OS) [15]. A number of clinical studies have demonstrated a relation between male infertility and OS [16-18]. Therefore the present study was carried out to provide substantial scientific data the possible effect of male age on the conventional semen parameters, together with sperm DNA fragmentation and total anti oxidative capacity (TAC) as a measure of OS.
Study population and sample collection
The study was approved by the ethical review board of Arabian Gulf University. Verbal and written consents were taken from the volunteers. All patients received an explanation of the study prior to obtaining informed consent. Clinical data was collected from patients by using a special questionnaire, designed for the purpose of the study. The questionnaire was designed to obtain information on, age, parity, past medical and surgical history, childhood diseases, socio-demographic characteristics, and drug history. Each patient was requesting to fill the questionnaire. Definite inclusion criteria were used to select patients who would be involved in this study; and they were including:
1. Scheduled for a routine semen analysis as a patient at Salmania medical complex
2. Age 18 years and more
3. Not currently receiving hormonal treatment.
The exclusion criteria were:
1. Incomplete semen samples
2. Incomplete questionnaire
A total of fifty two adult males; who fulfilled the criteria of inclusion were participating in this study.
Semen samples were collected from infertile male patients attending Salmania medical complex. All samples were collected at hospital lab by masturbation into sterile containers after 3-4 days of sexual abstinence. All semen analysis tests were performed immediately upon receipan by same person. After liquification, conventional analysis was performed according to World Health Organization guidelines [19]. The variables taken into account were volume of ejaculate (ml), round cells concentration (×106/ml), Sperm concentration (×106/ml), forward motility (%) and morphology (% of normal forms). A leukocyte count (×106/ml) was carried out by using standard peroxidase test. Sperm morphology assessment was performed according to Björndahl et al. [20].The semen samples were also analysis with flowcytometry and the colorimetric assay for (TAC).
Flow Cytometry (FCM) analysis
Sample preparation: Native sampled preparations of single cell suspension were performed according to the method of Ehemann et al. [21,22]. Semen samples were treated with two types of solutions: Ethanol fixation And Citric acid preparation.
Cytometry analysis of the fluorescence stained fixed samples: Flow cytometric analyses were performed using a CyFLOW space flow cytometer (PATREC, Germany) equipped with a UV laser diode and a 488nm argon laser and filter combination for 2,4-Diamidino-2- phenylindole (DAPI) stained single cells. The Multicycle program was used for histogram analysis; each histogram represents (3*104) cells for measuring DNA index. Human lymphocyte nuclei from healthy donors were used as internal standard for calibration the diploid DNA-index.
Analysis of frequency histograms: DNA frequency histograms were evaluated using cumulative frequency distributions ascribed by Hacker- Klomet al. [23]. The term (CC) was used to describe condensed chromatin and indicate haploid spermatozoa, which have a normal DNA content. Here, five categories could be recognized in each histogram: Cells with sub-haploid DNA content <1CC (debris that may be of apoptotic origin), mature haploid spermatozoa in the 1CC peak, haploid round spermatids in the 1C peak, diploid spermatozoa in the 2CC peak, cells registered to the right of the 2CC level including 2C cells (leukocytes, G1-spermatogonia, primary spermatocytes at preleptotene etc.), and to the right of 1CC which are cells in the DNA synthesis phase (S) and 4C cells (primary spermatocytes Presence of aneuploidy cells was marked as other bivarient histogram with blue colour. And the software was analysing the two types of population cells in the same diagram.
Total antioxidant capacity assay of semen (TAC)
Sample preparation: The samples were prepared according to Said et al. [24]. After liquefaction, aliquots (300 μl) of each sample were centrifuged at 1337 rpm for 7 minutes. The supernatant was aspirated and recentrifuged at 1337 rpm for another 10 minutes. The seminal plasma was frozen at -70°C until further use.
Colorimetric assay for TAC: The method was performed according to Mahfous et al. [15]. The frozen seminal plasma was thawed by putting the vials in an incubator at 37°C for 20 minutes and immediately assessed for its antioxidant capacity. TAC of seminal plasma was measured with the colorimetric method using the Cayman’s antioxidant assay kit (Cayman’s Chemicals Company, USA). All seminal plasma samples were diluted 1:10 with the assay buffer before assaying. All reagents and samples were equilibrated to room temperature before beginning the assay. Two observers assayed samples as well as Trolox standards twice. Trolox standards and reagent were prepared as per the manufacturer’s instructions at the time of the assay. A quantity of 10 μL of Trolox standard and samples were loaded on to the corresponding wells of a 96-well plate. Then 10 μL of metmyoglobin and 150 μL of chromogen were added to all standard/sample wells. The reaction was initiated by adding 40 μL of hydrogen peroxide as fast as possible. The plate was covered and incubated for 5 minutes on a shaker at room temperature. Absorbance was monitored at 750 nm using ELx800 Absorbance Microplate Reader.
Calculation of assay results
This was done by calculating the average absorbance of each standard and sample. The average absorbance of the standards as a function of the final Trolox concentration (mM) was plotted for the standards curve in each run, from which the unknown samples were determined. The total antioxidant concentration of each sample was calculated using the equation that had been getting from the linear regression of the standard curve by substituting the average absorbance values for each sample into the equation:
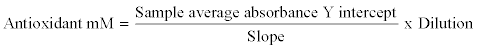
Accuracy and sensitivity of the TAC assay kit: According to the manufacturer, the assay precisions were: inter assay coefficient of variation 3% (n=20) and intra assay coefficient of variation 3.4% (n=84). The assay kit could measure samples containing antioxidants ranging between 44 and 330 m M without any further dilution.
Data analysis: Data analysis was performed using the SPSS 16 for Windows Software Package (SPSS Inc., Chicago, IL). Data were expressed as percentage and Mean ± SD. To find out the independent association between the different seminal parameters and selected variables, logistic regression analysis was carried out. Statistical significance was defined as p<0.05.
More than half of the patients 63.5% were in the age group 26-35 years. Around 21.2% were in the age group 18-25 years and (9.6%) were in the age group 36-45 years. The rest of patients 5.8% were above the age of 45 years. The age range was 21-52 years, with a mean age of years 30.63 years ± 6.7. The age distribution can be shown in (Figure 1).
Around 86.5% of patient presented with primary infertility while only 13.5 % were complaining of secondary infertility. Regarding their medical history; nearly 11.5% of the patients with male infertility had history of UTI, 3.8% gave history of sickle cell anaemia, 3.8% had Hypertensive disorders, and 1.9% had Diabetes mellitus. Only 5.8% of patients had history of pelvic surgery; of them about 3.8% were previously treated with pelvic surgery for inguinal hernia, and 1.9% had surgery for varicocele. Only 3.8% gave history of Mumps virus infection. Social history revealed that 42.3% were smokers, with no history of alcohol intake. According to their jobs; nearly half of the patients (51.9%) were occupied in moderate activity jobs, whereas (32.7%) were engaged in labor work. The remaining (15.4%) were had a sedentary type of work. Patients with secondary infertility tended to have less activity jobs in comparison to patients with primary infertility. No history of exposure to occupational hazard (radioactivity or organic solvent exposure). There was significant negative correlation between presence of UTI and semen parameters, also smoking had significant negative correlation with semen analysis (p<0.005).
The abnormality of semen samples in relation to patient’s age was analysed (table 1). There was a significant negative correlation between age of the patient and sperm count, vitality, motility and morphology.
| Age groups (years) | Concentration(million/ml) | Total motility (%) | Morphologically normal form | Vitility (%) |
|---|---|---|---|---|
| 18-25(n=11) | 45.18±22.66 | 45.74±14.55 | 48.00±13.83 | 56.45±12.05 |
| 26-35(n=33) | 40.93±22.54 | 36.35±16.26 | 34.11±11.17 | 40.19±12.80 |
| 36-45(5) | 11.65±9.76 | 18.92±14.94 | 22.58±8.24 | 23.67±4.71 |
| 45-55(3) | 7.73±2.46 | 8.33±1.52 | 16.67±2.05 | 13.67±2.51 |
| Test for trend(P) | <0.005 | <0.005 | <0.005 | <0.005 |
Table 1: The Mean ± SD of conventional semen parameters in relation to age groups.
As patients age increases, there was a significant reduction in sperms count (Figure 2). The sperm count declined from the youngest age group (18-25) where the mean of sperm count was (45.18 million/ml) to the older age group; where the mean of sperm count was (7.37 million/ ml). The mean percentage of sperm vitality also decreased with advance age, from (56.45%) in the younger age group, to reach (13.67%) in the older age group (Figure 3). Likewise, the mean percentage of motile sperms, declined with advance age, from (45.73%) in the younger age group, to reach (8.33%) in the older age group (Figure 4). Furthermore, the percentage of morphologically normal sperms was varied between age groups. In the youngest age group, the mean value was (48.0%), whereas in the older age group, the mean was (16.67%), (Figure 5). On the other hand the age of the patients had no significant relation to leukocyte count (p>0.05).
Another significant correlation was established between patient age and DNA integrity. There was increase in DNA fragmentation with increase patients’ age as shown in (Figure 6). In addition, the level of TAC in the patients’ seminal plasma was negatively correlated with advancing age. The mean level of TAC had been decline, from (1354.64 m mol/ml) in the youngest age group, to the lowest value, at the oldest age group (771.67 m mol/ml) (Figure 7).
The purpose of the study was to analyse the effect of increase male age on certain fertility parameters. Unlike women, who are fertile during specific period of their life that usually not exceed the age of (50) years, men can have children beyond the age of (40). There is no proven cause to limit the sperm production in men as they grow older as spermatogenesis continues into advance ages; however; age dependent alterations in male fertility have been documented. Several studies had demonstrated a decrease in semen quality with advance age and subsequent infertility [25-27].
The present study assesses the relationship between age of the patients and semen parameters of (52) infertile patients. All patients were aged between (18-55) years old. The sperm counts (million/ml), motility (percentage), and morphology (percentage) evaluation were determined according to WHO criteria. Sperm DNA integrity and TAC level of seminal plasma were also assessed.
The present study demonstrated significant associations between male infertility and patient’s age. The sperm counts were significantly decrease with the increase of patient’s age, and they were particularly lowest in the proportion of patients who were 45 years or above. The results of the present study, agreed with that of Ku¨hnert and Nieschlag [28]; who concluded that, advance male age has deteriorated effect on sperm count, particularly after the age of 40.
The mean percentage of sperm vitality also decreased with advance age. This result agreed with that of Molina et al. [29] who found that a significant decrease in seminal volume, sperm count, motility, viability and normal and morphology in relation to age.
Furthermore, a significant correlation was also established between sperm motility and patients’ age. The percentage of motile spermatozoa, were lower in oldest age group (45-55) than the rest of patients and this was also illustrated by studies conducted by Tingen et al. [26], and Sloter et al. [27] who concluded that the percentage of progressively motile sperm decease as patient getting older especially after late-thirties.
Another significant correlation was established between sperm morphology and patients age. The percentage of morphologically normal spermatozoa, were higher in younger age group (below 30) than older patients, and this finding was also revealed by studies conducted by Chen et al. [30], Eskenazi et al. [6] who found significant increase in the percentage of abnormal spermatozoa with advancing age.
However, age of the patients had no effect on the number of leukocytes in semen samples. The present study showed no statistically difference in the number of leukocyte in the semen samples of the patients in the study group. This result agreed with Lackner et al. [31] who concluded that the age by itself has no effect on presence or absence of leukocyospermia.
Male aging was positively related to the level of DNA fragmentation assessed by cytomertry analysis of sperm chromatin condensation. This relation can be attributing to ageing process consequence with defect in the control of spermatogesis. These defects can result in infertility, pregnancy loss or even malformed offspring [32,33].
Nevertheless, age had no effect on the level of aneuploidy changes that were observe in the semen of the study group. This agreed with Schmid et al. [32] and Wyrobek et al. [34] who concluded that, there were no consistent correlations between advancing age and aneuploidy rate of sperm in infertile male patients.
The level of antioxidant substances that were measured by TAC assay method were negatively correlated to patients’ age. This result can be attributed to age effect as supported by, Cocuzza et al. [35] and Fingerova et al. [36], findings which revealed that older age males have higher level of ROS and intern low level of TAC and these render older males less fertile than the others do.
In conclusion, our data showed that increasing in age significantly influences semen parameters required for healthy male fertility. Thus, men choosing to delay fatherhood may have a lower likelihood of a successful pregnancy free of early loss and gene defects.