Journal of Glycobiology
Open Access
ISSN: 2168-958X
ISSN: 2168-958X
Research Article - (2021)Volume 10, Issue 6
Plants adapt and survive adverse environmental changes by altering their membrane lipid composition. Sesuvium portulacastrum L., a facultative halophyte has been extensively studied for its adaptability to various abiotic stresses. However, knowledge on the identification of glycerolipids and changes in their profile in coping up with the stressors is limited. In this investigation, electrospray ionization tandem mass spectrometry (ESI‐MS/MS) was used for identifying glycerolipid molecular species in the halophyte and to study changes in their profile during pre- and post monsoon seasons. The study showed when environmental conditions changed from post to premonsoon season, the lipidome switched from galactoglycerolipids, such as monogalactosyl diacylglycerol (MGDG, GL1), lyso-digalactosyl diacylglycerol (lyso-DGDG,GL2) and digalactosyl monoacylglycerol phosphatidic acid (DGMAPA, GL3) to MGDGs (GL1;GL1a), monogalactophosphatidyl glycerols (MGPGs, GL4-GL7) and sulfonoglycolipids, (SQMG, GL8) and (SQDGs, GL9 and GL10). Glycolipids GL1-2 and GL8-10 are reported earlier while glycerophospholipids (GL3- GL7) are new natural products. The degree of fatty acid unsaturation of polar lipid fraction decreased with stronger abiotic stresses. Polyhydroxy nature of glycerolipids played a protective role against oxidative damage during harsh condition by acting as oxygen radical scavengers. Saturation of the fatty acyl chain contributed to the homeostasis of membrane fluidity and permeability.
Aizoaceae; ESI-MS; Glycerolipids; Halophyte; Structure
Exposure of plants to abiotic stresses causes them to undergo significant alteration in their membrane lipid composition. During acclimation to environmental changes there is an increase in the phospholipid content and degree of fatty acid saturation. There is an enhancement in cellular functions and membrane integrity caused by changes in the lipid content to undergo non‐bilayer phase formation [1]. Additionally, it is known that membrane lipids can be excellent sources for generation of lipid mediators. According to, lipid mediators like free fatty acids, lysophospholipids and phosphatidic acids participate in numerous cellular processes in plant stress responses. Some of the plant stress responses include cytoskeletal reorganization, vesicular trafficking and signal transduction [2]. Hence, it may be concluded that changes in the membrane lipid composition due to environmental changes can play regulatory and structural roles in plant adaptation including their survival.
Plants growing in highly saline soils and possessing exceptional ability to tolerate high salinity conditions are halophytes. In order to survive under high saline environment these plant species act as salt excluders, and thus efficiently manage ion homeostasis through transporters back from leaf into roots or sodium sequestration in vacuole [3]. Under saline conditions, halophytes use Na+, Cl-, and K+ for maintenance of shoot osmotic pressure [4]. Other compatible solutes like proline, glycine betaine, sugars, phenolics as well as antioxidant enzymes (superoxide dismutase, catalase, guaiacol peroxidase, ascorbate peroxidase, and glutathione reductase) and non-enzymatic antioxidants (like ascorbate and glutathione) also play an important role in the adaptation mechanism of halophytes [5].
Sesuvium portulacastrum L. (Aizoaceae), commonly called ‘sea purslane’, is a psammophytic facultative halophyte naturally growing in the tropical and subtropical brackish areas around the world. This plant species has a distinct molecular and physiological flexibility that enables it to adapt and survive under various abiotic constraints such as salinity, drought and heavy metal contamination [6]. As a naturally salt tolerant plant species with an interest for sand dune fixation, desalination and phytoremediation along coastal regions, S. potulacastrum plants show considerable economical and medicinal potential.
Several research groups working with plant biochemical and physiological studies showed how various parameters like enzymes (V-ATPase, H+ ATPase and F- ATPase), osmolyte accumulation and antioxidant defence are playing important role under salt stress conditions [7]. However, there is limited information about biochemical characteristic of the glycerolipids as well as salt-stress induced variation in their chemical composition in the halophyte. Therefore, relationship of glycerolipids with physiological reactions of plants still remains unknown.
Analysis of membrane lipids have been technically challenging as lipids comprise of diverse chemical compounds belonging to three major groups: glycerolipids, sphingolipids, and sterols. Glycerolipids have glycerol backbone with two fatty acid (FA) molecules bound at sn1 and sn-2 positions and can be classified into head‐group classes depending upon the substituent at the sn-3 position with either sugar (glycolipids) or phosphorous (phospholipids). Each class is composed of various molecular species, as the fatty acids or other hydrocarbon portions vary in chain length and unsaturation. Actually purified from biological source glycerolipids are often mixtures difficult to separate into each molecular species. Although several sensitive techniques have been developed, electrospray ionization tandem mass spectrometry (ESI-MS/MS) has emerged as a powerful tool for the rapid analyses of unresolved mixtures of complex molecules especially present at very low levels encountered in biological samples.
According to the regular patterns of photosynthetic polar lipid standards mass spectrometry, different lipid classes produce characteristic fragment ions in the positive or negative mode which is consistent with the results of other scientists [8]. Tandem mass (MS/MS) generates ions characteristic of the structure of the polar head group as well as the fatty acid components including their attachment at the glycerol molecule (sn-1, sn-2 /sn-3). Further, the abundance of the fragment ions produced by the cleavage of the fatty acyl groups is governed by steric hindrance [9]. Therefore, in the positive mode the product ion (m/z 243) was used to diagnose the specific class of MGDG and signal resulting from loss of 342 amu/the presence of peak at m/z 325 is an indication of terminal digalactosyl at sn-3 position in DGDG. The presence/elimination of ion pair at m/z 81 [HPO3+H] + and m/z 97 [HPO4+H] +; the daughter ion at m/z 199 corresponds to disodiated five or six membered hydroxyl cyclophosphane and the fragment at 171 amu /153 (171-H2O) are characteristics of phosphatidyl glycerols (PG) [10,11].
Published articles revealed that sulfolipid production in S. portulacastrum was enhanced under salt stress conditions, whereas, an increase in the accumulation of the major glycolipid monogalactosyl diacylglycerol (MGDG, GL1) (16:0/18:3) was observed under low level of salt treatment [12,13]. Further, cadmium stress caused a decrease in the content of total lipids and their fractions including galactolipids, phospholipids and neutral lipids with no significant changes in fatty acid (FA) composition of S. portulacastrum [14]. There is solitary evidence in published reports on the variation in free amino acids and mineral constituents of S. portulacastrum plants over two seasons i.e. winter and summer [15]. Herein, we describe an ESI‐MS/MS based platform for characterisation of glyco-, phospho- and sulfolipid constituents. Changes of glycerolipid molecular properties in plants of S. portulacastrum in acclimation response to environmental conditions prevailing during pre- and post-monsoon seasons were analysed.
Application of ESI-MS and MS/MS offers a window to the comprehensive analysis of glycerolipids pattern under the two different environmental conditions. Figure1A depicts positive ion ESI-MS glycerolipids profile of PF1 (post-monsoon) and Figure1B illustrates glycerolipids profile of PF2 (pre-monsoon) is shown in Figure 1. PF1 exhibited molecular ions peaks at m/z 775.5818, m/z 649.2835 and 925.5597 and PF2 contained signals due to molecular ions at 601.1585, 611.1750, 627.1268, 669.174, 685.260, 753.3076, 755.2286, 823.3210 and 839.3132. It is important to note that m/z values with four decimal points were rounded off to zero decimal points. The theoretical mass expected based on the molecular formula is given in Table-S1. The chemical structures present in both the fractions are shown in Figure 2 were elucidated by ESI-MS/MS analysis of the molecular ions exhibited in the positive ion ESI-MS (Figures 1A and 1B). The fragmentation pattern observed in the collision induced dissociation (CID) or MS/MS spectra Fig. S1 (A-C), S2 (A-E)] is as given in Table 1 and schematically discussed in Figure 3. The analyses of PF1 based on ESI technique led to the structural identification of two known glycolipids (GL1, GL2) and a novel phospholipid (GL3); PF2 contained known glycolipids (GL1, GL1a and sulfonoglycolipids (GL8-GL10) besides four new glycerophospholipids (GL4 - GL7) is shown in Figure S1 and Figure S2. The identification of GL1a, GL9 and GL10 is based solely on molecular mass is given in Table S1.
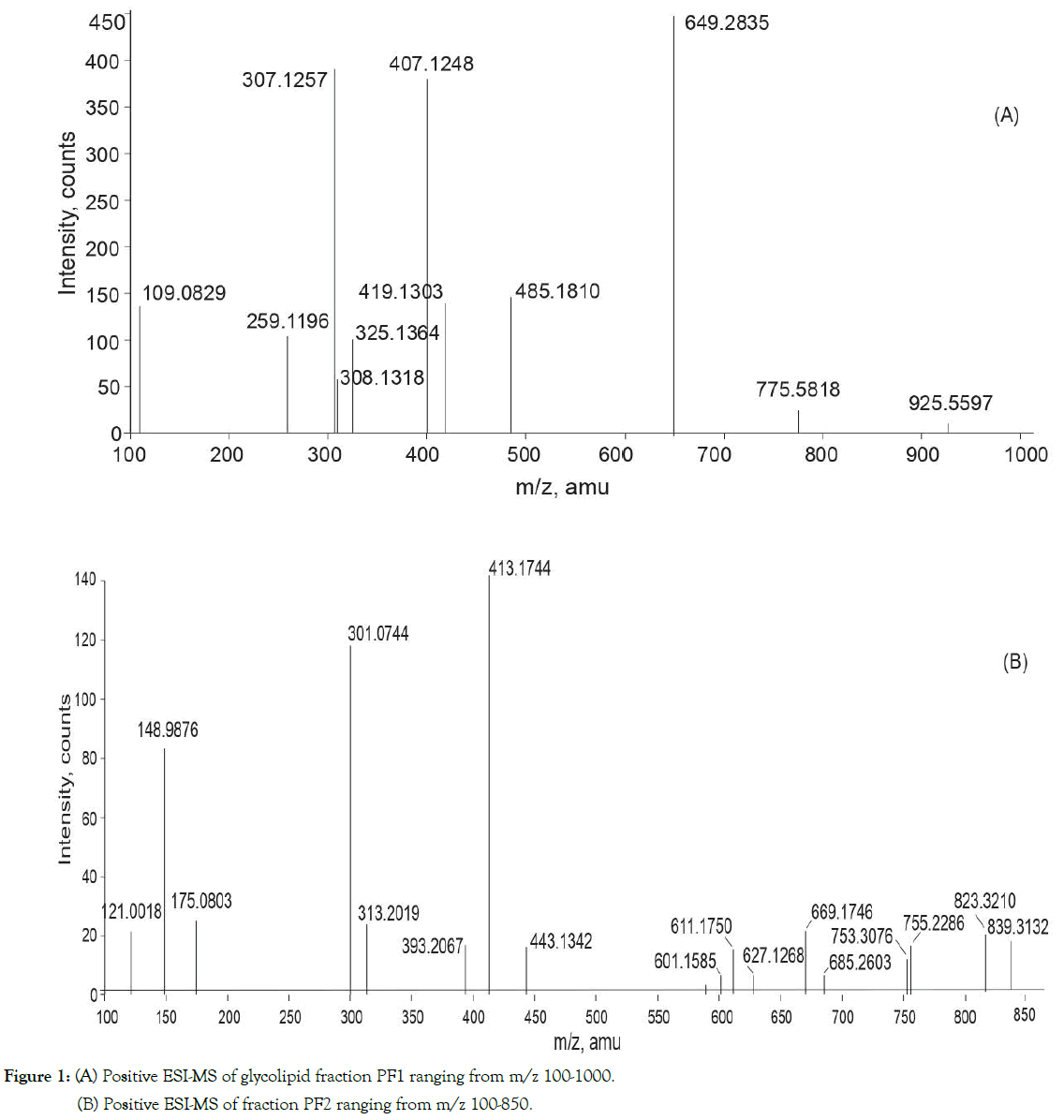
Figure 1. (A) Positive ESI-MS of glycolipid fraction PF1 ranging from m/z 100-1000.
(B) Positive ESI-MS of fraction PF2 ranging from m/z 100-850.
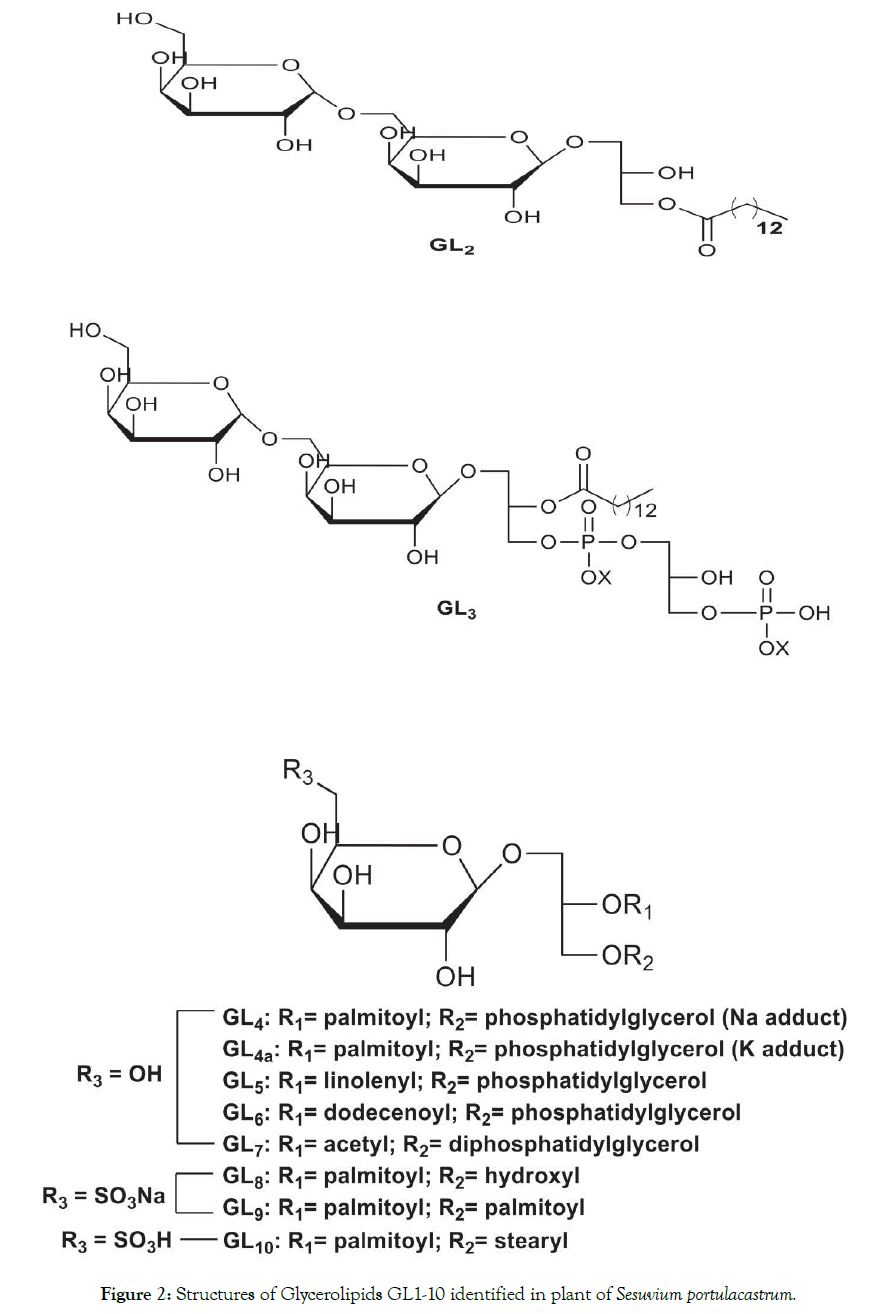
Figure 2. Structures of Glycerolipids GL1-10 identified in plant of Sesuvium portulacastrum.
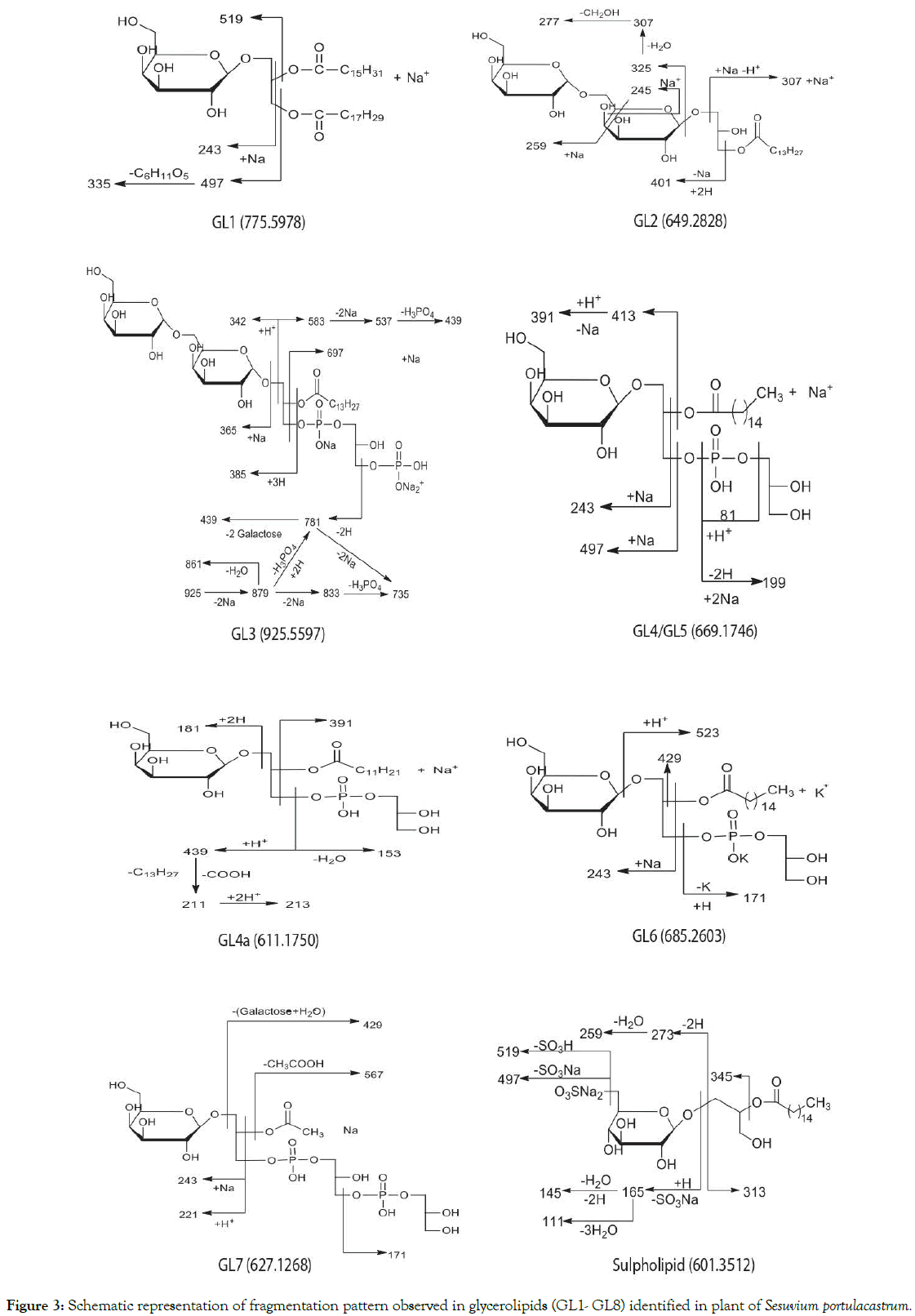
Figure 3. Schematic representation of fragmentation pattern observed in glycerolipids (GL1- GL8) identified in plant of Sesuvium portulacastrum.
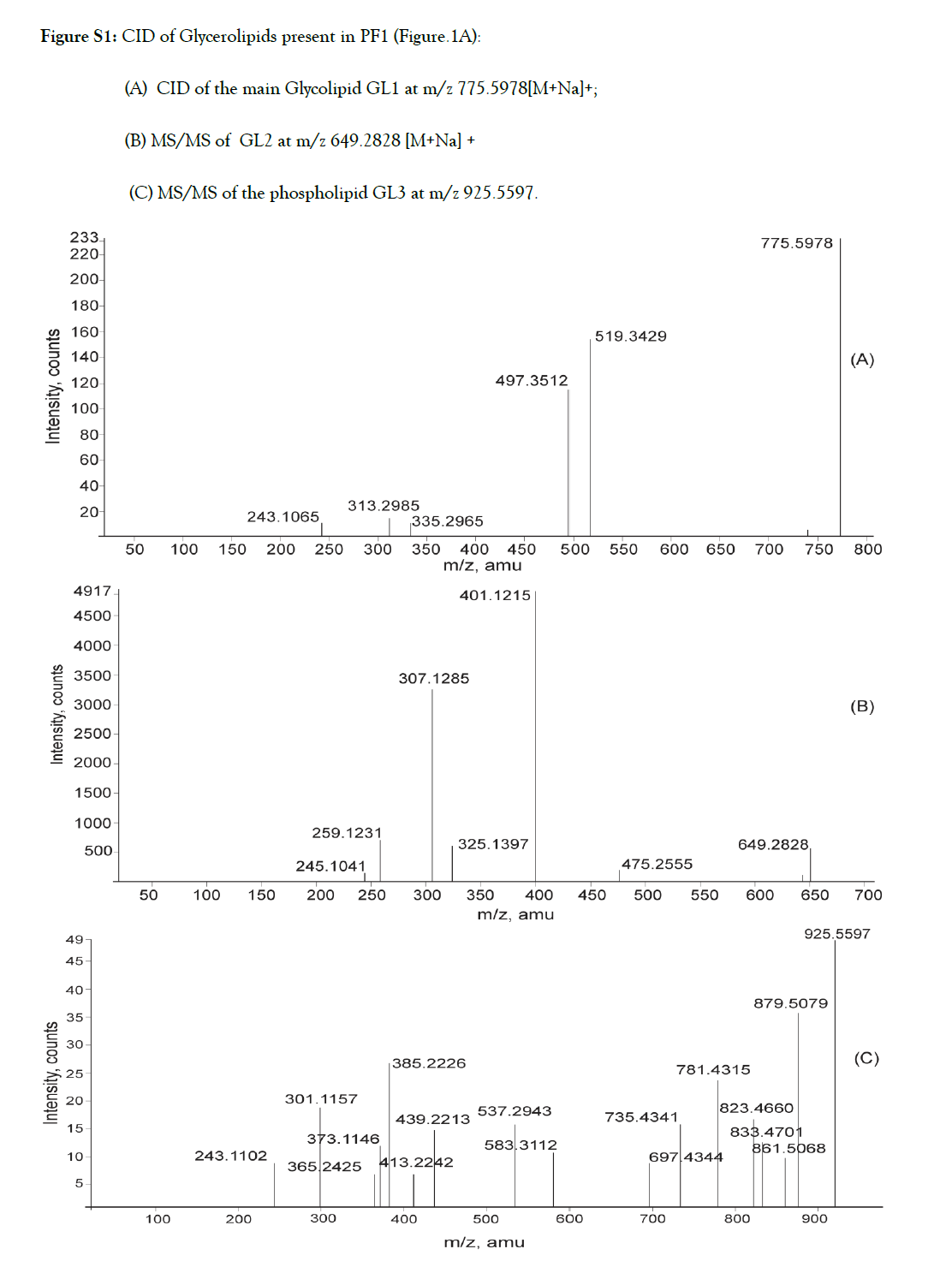
Figure S1. CID of Glycerolipids present in PF1 (Figure.1A):
(A) CID of the main Glycolipid GL1 at m/z 775.5978[M+Na]+;
(B) MS/MS of GL2 at m/z 649.2828 [M+Na] +
(C) MS/MS of the phospholipid GL3 at m/z 925.5597.
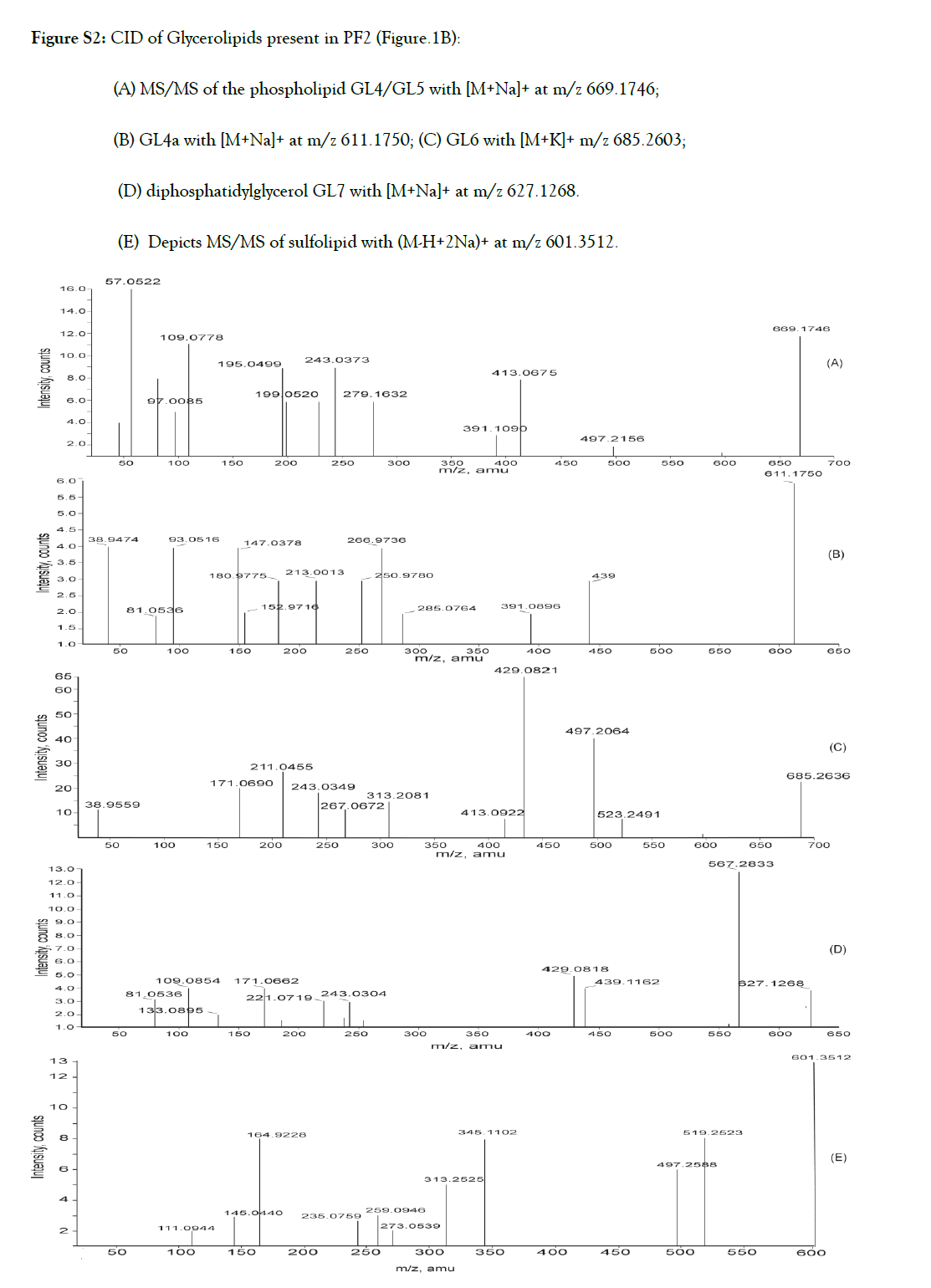
Figure S2. CID of Glycerolipids present in PF2 (Figure.1B):
(A) MS/MS of the phospholipid GL4/GL5 with [M+Na]+ at m/z 669.1746;
(B) GL4a with [M+Na]+ at m/z 611.1750; (C) GL6 with [M+K]+ m/z 685.2603;
(D) diphosphatidylglycerol GL7 with [M+Na]+ at m/z 627.1268.
(E) Depicts MS/MS of sulfolipid with (M-H+2Na)+ at m/z 601.3512.
| Lipid class | Structural Identification | Molecular ion/Fatty Acids Fragmentation observed in ESI-MS/MS Adduct PF1-Post-monsoon |
Fatty Acids | Fragmentation observed in ESI-MS/MS Fig. S1 (A-C), S2 (A-E) |
|---|---|---|---|---|
| MGDG (GL1) | 2R)-1linolenyl-2palmitoyl)-3-b-D-galactopyranosyl-sn-G | 775/Na+ | 18:3/16:0 | 519,497,335, 313, 243 |
| lyso-DGDG(GL2) | 3-Digalactosyl-1-myrsitoyl-sn-G | 649/Na+ | 14:0 | 475,401,325, 307,259,245 |
| DGMPGPA(GL3) | 3-Digalactosyl 2-myristoyl-1-sn-PG-PA | 925/Na+ | 14:0 | 879,861,833,823, 781, 735,697,583,537,439,385,365, 243 |
| PF2-Pre-monsoon | ||||
| MGDG (GL1) | (2R)-1linolenyl-2palmitoyl-3 b-D-galactopyranosyl-sn-G |
753/ H+ | 18:3/16:0 | Not fragmented |
| MGDG (GL1a) | 1linoleyl-2palmitoyl-3- galactopyranosyl-sn-G | 755/H+ | 18:2/16:0 | Not fragmented |
| MGMAPG (GL4) | 1-galactosyl -2-palmitoyl-sn-G-3PG | 669/Na+ | 16:0 | 497 ,413,391,279,243,199,195,109,97 |
| MGMAPG(GL4a) | 1-galactosyl -2-palmitoyl-sn-G-3PG | 685/K+ | 16:0 | 523,497,429,413,313,26 7,243,211,1 71 |
| MGMAPG (GL5) | 1-galactosyl -2 linolenyl -sn-G-3PG | 669/Na+ | 18:3 | 391,279 (present in the MS/MS of GL4) |
| MGMAPG (GL6) | 1-galactosyl -2dodecenoyl -sn-G-3PG | 611/Na+ | 12:1 | 439,391,285,267, 251,213, 181, 153,147,93,81 |
| MGMADPG (GL7) | 1-galactosyl -2acetyl-sn-G-3DPG | 627/Na+ | 2:0 | 567,439,429,243,221,1 71, 133,109,81, |
| SQMG (GL8) | 2-palmitoyl-3-(6′-sulfoquinovopyranosyl)-G | 601/Na+ | 16:0 | 519,49 7,345,313,2 73, 259, 235,165,145,111 |
| SQDG(GL9) | 1,2-dipalmitoyl-3-(6′-sulfoquinovopyranosyl)-G | 839//Na+ | 16:0/16:0; | Not fragmented |
| SQDG (GL10) | 1-steryl-2-palmitoyl-3-(sulfoquinovosyl)-G | 823/H+ | 18:0/16:0 | Not fragmented |
Table-1:Structural identification of glycerolipids molecular species and their profile in Sesuvium portulacastrumunder two different environmental conditions.
| Compound | Molecular Formula |
Theoretical mass(Da) |
Observed mass(Da) |
Mass relative Error(ppm |
|---|---|---|---|---|
| GL1 | C43 O10H 76.Na | 775.5338 | 775.5818 | 0.0480 |
| GL1a | C43 O10H 78.Na | 755.5675 | 755.2286 | 0.3389 |
| GL2 | C29O14H 54.Na | 649.3411 | 649.2835 | 0.0576 |
| GL3 | C32O22H 58P2.3Na | 925.2588 | 925.5597 | 0.3009 |
| GL4 | C28O14H 55P.Na | 669.2202 | 669.1746 | 0.0456 |
| GL4a | C28O14H 55P.K | 685.4289 | 685.2603 | 0.1686 |
| GL5 | C28O14H 56P | 669.2202 | 669.1746 | 0.0456 |
| GL6 | C24O14H 45P.Na | 611.2445 | 611.1750 | 0.0695 |
| GL7 | C17O19H 34P2.Na | 627.1066 | 627.1268 | 0.0202 |
| GL8 | C25 O11H 47S.Na2 | 601.2635 | 601.1585 | 0.1050 |
| GL9 | C43 O12H 83S | 823.5607 | 823.3210 | 0.2397 |
| GL10 | C41 O12H 77S Na2 | 839.4933 | 839.3132 | 0.1801 |
Table-S1:Molecular formulas and accurate mass measurements of the Glycerolipids obtained by High resolution MS experiments.
Profiling and tandem mass spectrometry of glycerolipids from S. portulacastrum of post monsoon collection (PF1)
Glycolipid GL1 [α]D = -6.95˚C (c= 0.58, MeOH) obtained as described in the Materials and Methods section, was identified as (2R)-1O-linolenyl-2O-palmitoyl–3O- D-galactopyranosyl-snglycerol. It is the major glycerolipid in samples of S. portulacastrum and also reported to be the most abundant glycolipid in cyanobacterium Synechocystis sp. PCC 6803. Availability of sufficient material led us to elucidate its complete structure by assigning all chemical shifts via analysis of 1D and 2DNMR data Table S2. The structure was confirmed by tandem mass analysis (Table I, Figure 3).
| Carbon No. | 1HNMR (δH, ppm) | 13CNMR(δc, ppm) | HMBC Correlation |
|---|---|---|---|
| Glycerol- 1a 1b 2 3a |
4.36(d, 9.9Hz) 4.21(d, 6.6Hz) 5.28 m 3.71(d, 6.6Hz) 3.89 (d, 4.5Hz) |
62.8 62.8 70.1 68.0 |
- - - - |
| Galactose- 1′ 2′ 3′ 4‘ 5‘ 6‘ |
4.23(d, 7.2 Hz) 3.60 3.88 3.71 3.66 3.89,3.91 |
103.9 71.1 68.9 74.5 73.3 61.5 |
- - - - - - |
| Fatty acids-1“ 2“ 3“ 4”- 7 8” 11”, 14′’ 9”, 10”,12”, 9”, 10”,12”, 13”, 15”, 16” |
- 2.30(d, 4.2Hz) 1.58 1.28 2.05 2.78(t, 5.7Hz) 5.31-5.33 (Cluster) |
173.8 34.2 25.4 29.1-29.6 27.1 25.5 127.0-131.9 (6d) |
- - 3”,1”,4” 2”,4” 3”,5” 9”, 10” - |
| 17” | 1.23(s) | 31.8 | 18” |
| 18” | 0.95(t, 7.5Hz) | 14.2 | 17” |
| 1’’’ | - | 173.4 | - |
| 2’’’-3’’’ | 2.03-1.58(m) | 24.8-27 | - |
| 4’’’-15’’’ | 1.28 | 22.5-25.5 | - |
| 16’’’ | 0.87(t, 6.9Hz) | 14.0 | - |
Table-S2: 1H and 13CNMR data of glycolipid GL1.
CID of [M+Na]+ ion of GL1 at m/z 775 (Table I, Fig.S1A) generated two dominant fragments via loss of palmitic acid (C16:0 from the sn-2 position) as the main ion at m/z 519 and a less intense peak at m/z 497 due to the loss of linolenic acid (C18:3from sn-1 position). These results were suggestive of loss of palmitic acid in position sn-2 being favoured for steric reasons. Fragment ion at m/z 243 is characteristic of MGDG.
Fragmentation of [M+Na]+ ion at m/z 649 (Table I, Figure 3, Fig. S1B) gave diagnostic signals at m/z 307 resulting from the loss of 342 amu indicative of digalactosyl head group (sn-3) and the elimination of myristic acid (C14:0) from the sn-1position yielded the most abundant ion at m/z 401. The positive ions at m/z 245 and m/z 259 are indicative of (1→6) sugar linkage between the two sugar units. The dissociation pathway is consistent with a lysoglycerolipid structure, 3-O-digalactosyl-2-hydroxy-1-O-myrsitoylglycerol (GL2).
Analysis of the product ion spectrum of parent molecule at m/z 925 (Table I, Figure 3, Fig.S1C) showed three quasi-molecular ions for the glycerophospholipid [m/z 925 (M++4Na+H), m/z 879 (M++2Na+H) and m/z 833 (M++H)] each giving rise to specific fragmentation pattern (Figure 3).
CID of the ion at m/z 925 (M++4Na+H) produced fragment at m/z 583 with the neutral loss of 342 amu suggestive of digalactosyl head group further confirmed by the presence of the sodiated digalactose signal at m/z 365. Elimination of myristic acid (C14:0) from sn-2 position of glycerol generated ion at m/z 697. Dissociation of digalactosyl head group together with disodium salt of phosphoric acid (Na2HPO4) resulted in diagnostically important daughter ion at m/z 439. Alternately, simultaneous elimination of fatty acyl group as well as diphosphatidyl glycerol moiety also led to the formation of the sodiated digalactosyl glycerol at m/z 439. Elimination of 2 Na atoms from the parent ion generated ion at m/z 879 which lost dihydrogeno-phosphate group to produce peak at m/z 781. Direct elimination of (NaHPO3) group from the parent molecule resulted in the signal at m/z 823. Fragment at m/z 879 generated ions at m/z 833 on further loss of 2Na and m/z 861 on removal of one water molecule. These features in the MS/MS spectrum, as shown in Figure-3, identified the glycophospholipid GL3 as 3-O-digalactosyl- 2-O-myristoyl-1-sn-phosphatidyl-1′-sn-glycerol-3′phosphonic acid. This compound is a new natural product.
The number of Na+ ions in the phospholipids is known to alter its fragmentation pattern and it has been suggested that the sample be supplemented with NaOH solution to avoid formation of multisodiated ion, making the positive mass spectrum simpler and easier to interpret [16]. In the present investigation this was not possible because the multisodiated molecular species GL3 is a minor constituent, in the glycerolipid fraction PF1.
Sulfoquinovosyl diacyl glycerols (SQDGs) are negatively charged at neutral pH. Hence, they are expected in admixture with galactoglycerolipids as sodium adducts. Unfortunately, no
Profiling and tandem mass spectrometry of glycerolipids from plants of S. portulacastrum of pre-monsoon collection (PF2)
The positive ion ESI-MS spectrum (Figure 1B) of PF2 exhibited protonated /sodiated/potassium adducted molecular ions signals at m/z 611, 627, 669, and 685 compatible with phosphatidyl glycerol structures. Protonated ions [M+H] + at m/z 753 and m/z 755 were assigned to MGDG’s with 16:0/18:3 (GL1of post monsoon collection) and (GL1a) 16:0/18:2 fatty acid composition respectively. Additional signals observed at m/z 601, m/z 823 and m/z 839 were assigned to sulfolipids esterified with palmitic acid (SQMG, 16:0, GL8), SQDG (16:0/16:0, GL9) and GL10 esterified by palmitic acid and stearic acid, SQDG (16:0/18:0, GL10) respectively. By using the MS/MS information we were able to decipher the structure of components of PF2 (Table I and Figure 3).
Dissociation features observed in the MS/MS spectra (Table I, Figure 3, Fig.S2 (A-E)) of molecular ions at m/z 611, 627, 669, and 685 indicated that they are likely to be monogalactosyl monoacyl phosphoglycerols. Thus, the signals at m/z 81 and m/z 97 (H3PO4), the daughter ion at m/z 199 (disodiated five or six membered hydroxyl cyclophosphane) and the fragment at 171 amu /153 (171-H2O) are characteristics of phosphatidyl glycerols (PG). The fragment ion at m/z181 due to galactose and ions at m/z 221and m/z 243 were suggestive of a monogalactosyl moiety at the sn-1 position. The ions at m/z 669 and 627 produced fragments at m/z 413 (M+-C16:0) and m/z 567 (M+- C2:0) respectively due to elimination of palmitic and acetic acids from the sn-2 position in these glycerolipid molecules. The spectrum of ion at m/z 611 was indicative of C12:1 acyl chain at the sn-2 position of glycerol. The aforementioned fragmentation is compatible with the structure of 1-O-galactosyl -2-O-palmitoyl-sn-glycerol-3-phosphoglycerol for GL4, 1-O-galactosyl -2-O-dodecenoyl-phosphatidyl glycerol for GL6 and 1-O-galactosyl-2-O-acetyl-3-diphosphatidyl glycerol for GL7.
The product ion spectrum of the phospholipids with parent ion at m/z 669 (Table 1, Figure S2A) also contains a signal at m/z 391 resulting from elimination of 278amu which is equivalent to the molecular mass of linolenic acid. The peak at m/z 279 confirmed the presence of the corresponding fatty acid. This indicated that the glycerolipid with 669amu molecular mass is a mixture of galactophospholipids GL4 and GL5 with palmitic acid and linolenic acids respectively at the sn2 position. GL4 is a sodiated adduct ion of 1-O-galactosyl-2-O-palmitoyl-sn-glycerol- 3-phosphoglycerol and GL5 is the protonated molecular ion of 1-O-galactosyl-2-O-linolenyl-sn-glycerol-3-phosphoglycerol.
The glycerolipid with molecular mass of 685.2603 amu is the potassium adduct of 1-O-galactosyl-2-O-palmitoyl-sn-glycerol-3- phosphoglycerol (GL4a).
The results (Table I) of the present investigation revealed that GL1 is the major glycolipid of the halophyte, in both the seasons. PF1 (Figure 1A), also contained considerable amount of lysodigalactosyl monoacylglycerol (GL2) while (GL3) was a novel minor phosphoglycerolipid. The premonsoon sample, PF2 (Figure1B) showed the presence of novel glycophosphatidyl glycerols (GL4- GL7) and sulfonoglycolipids, (GL8)-(GL10) as minor constituents in addition to MGDGs, GL1(16:0/18:3) and GL1a (16:0/18:2). No SQDG was identified in PF1 while PF2 was devoid of DGDG.
It is worth mentioning here that Ramani reported identification and enhancement of sulfolipids 16:0/18:3 and 18:3/18:3 under salt stress. However, under salt stress, GC-MS analysis of SQDGS fatty acids composition from the halophyte showed presence of stearic, oleic, linoleic acids besides linolenic and palmitic acids. Though not reported by the authors, it is clear that the halophyte must also be producing sulfolipids containing stearic, oleic and linoleic acids.
It is well known that halophytes have certain adaptation mechanisms to maintain the required liquid phase state of cell membranes under harsh conditions. FAs of polar lipids of photosynthetic tissue membranes undoubtedly play an important role in order to preserve their vital functions. Present findings show that with increase in salinity, temperature and humidity there was an increase in the saturation of (C14:0; C16:0 and C2) of fatty acyl groups, and the degree of FA unsaturation of neutral glycolipids (MGDGs) decreased as evidenced by the substitution of linolenic acid in (GL1) by linoleic acid in (GL1a). Except GL5, which was detected in admixture with GL4 and found to contain unsaturated linolenic acid, glycophosphatidyl glycerols, with the exception of GL6 which showed the presence of dodecenoic (C12:1), contained mainly saturated, myristic (C14:0), palmitic (C16:0) and acetic (C2:0) acids. Similarly, sulfolipids also contained exclusively saturated stearic (C18:0), myristic (C14:0) and palmitic (C16:0) acids. SQDG is the most saturated class in all species of marine macrophytes [17].
Most probably, it is for the first time in this paper that a detailed comprehensive research demonstrating profiling of cellular glycerolipids was made for two different environmental conditions in the halophyte. Additionally, glycerolipids, GL3-GL7, are new to the literature.
Plants when exposed to different abiotic stresses undergo alterations in the membrane lipids. These alterations could be due to variation in the nutritional resources, stress due to change in salinity, light, and/or temperature. It is therefore quite natural to find changes in the lipid composition with seasonal variation. Furthermore, membrane lipid changes can cause a direct effect on the membrane protein properties, activities of signalling molecules, fluidity and permeability adjustments of membranes as well as activating signal transduction pathways.
Glycerolipids constituting the matrix of photosynthetic membranes, appear to be concentrated within the chloroplast and endoplasmic reticulum. There are two major categories of glycerolipids depending on the nature of a hydrophilic head: (i) phosphoglycerolipids, being the main components of extrachloroplast membranes and (ii) galactolipids, which are the main building blocks of plant chloroplasts [18]. In order to maintain activities of key proteins associated with photosynthesis as well as membrane stability it is necessary that balance between the bilayer forming galactolipids, DGDGs and non-bilayer forming MGDG along with suitable quantity of acidic SQDG and PG in the chloroplast is required. The acidic lipids are known to substitute each other to maintain the amount of total anionic lipids in the thylakoid membrane, with PG having indispensable functions in photosynthesis.
In thylakoids, the relative proportions of different glycerolipids are stable, indicating the presence of mechanisms establishing and controlling the biological system to maintain stability while adjusting to conditions that are best for its survival. The thylakoid lipidome results from the activities of biosynthetic enzymes that generate each lipid, the trafficking of lipid intermediates, the catabolic pathways and the regulatory processes that ensure that appropriate proportions are reached [19].
Changes in the chemical composition induced by abiotic stress/ environmental perturbations, particularly glycerolipid composition, are not well understood and their relationship with physiological status remains to be studied. Most of studies, up to now, are focused on the influence of experimentally induced stressors under controlled conditions. Earlier studies report identification and enhancement in 16:0/18:3 and 18:3/18:3 SQDG content under high salinity, in plants of S. Portulacastrum in order to stabilize ATPase complexes and photosystem I (PSI) activity. It also states 1.2 fold increased accumulation of glycolipid GL1 under low salinity conditions.
Present study deals with the identification of glycerolipids and their profiling in S. portulacastrum plants during pre- and post-monsoon. The analysis (Table 1) clearly shows that there is a significant impact on lipid metabolism in the plant and the changes observed have helped us in understanding the adaptation mechanism of the halophyte. As evident from the results, the glycerolipid profile of PF1 (Table 1) showed to contain digalacto-phosphatidic acid (GL3), a precursor metabolite for phospho-glycerolipids and galacto-glycerolipids, as a minor constituent with saturated myristic acid (14:0) as acyl substituent at sn-2 position. Although they are usually very low in abundance, signaling lipids can be synthesized and accumulated quickly, from pre-existing membrane lipids or intermediate products of lipid biosynthesis, in response to adverse environmental conditions [20]. Several studies have demonstrated an accumulation of the lipid secondary messenger phosphatidic acid (PA) in responses to a wide array of abiotic stress stimuli, including salt stress [21]. PA can be either formed directly through hydrolysis of phospholipids by phospholipase D (PLD), or indirectly via sequential action of phospholipase C (PLC) and diacylglycerol (DAG) kinase [22,23]. Stimulated PLD activities are known in plants subjected to salt stress, which include cell cultures of Chlamydomonas reinhardtii, seedlings of A. thaliana, and tomato cell suspensions, resulting in increased PA levels [24,25].
No sulfolipids were detected in the post-monsoon sample. Environmental and nutritional factors like water deficiency, temperature, sulfate deprivation, and phosphate starvation are known to influences the synthesis of SQDG [26-29]. In the present investigation, sulphate deprivation seems to play a role as the other factors, namely, water deficit, temperature and salinity are less likely to affect sulfonolipid production under the environmental conditions prevailing during post-monsoon season. Further, it has been suggested that the two acidic lipids, sulfolipids and phospholipids, substitute for each other under some particular physiological conditions. Bacteria, Rhodobacter sphaeroides, cyanobacteria, Synechococcus elongates PCC7942, algae Chlamydomonas reinhardtii and higher plant species Arabidopsis thaliana, when grown under phosphate limitation, decreased PG content with accumulation of SQDG [30].
Similarly, when starved of sulphate, Chlamydomonas reinhardtii decreased the content of the sulphur containing lipid, to an almost undetectable level with a quantitative increase in the PG content. Overall, the acidic lipids seem to replace each other for the functioning of the photosynthetic apparatus as much as possible even under physiologically aberrant conditions that induce the loss of one acidic lipid [31]. This is well in agreement with the present observation wherein post-monsoon fraction PF1, contained phosphatidic acid, and no sulfolipids.
In plants of S. portulacastrum under abiotic stress (Table 1, PF2) lower production of MGDG (26.2 mg) was associated with the reduction of unsaturation wherein linolenic acd (18:3) in GL1 was partially substituted by linoleic acid (18:2) as in GL1a. The observed accumulation of 18:2 acyl component in GL1a under stress does not exclude an alteration of FA desaturase activity. However, in fraction PF2, though the profile was rich in acidic phospho-(GL4-GL7) and sulfolipids (GL8-GL10), it was devoid of DGDG. Under nutrition starvation and stress conditions, DGDG is exported to various extra-plastid membranes substituting for phosphoglycerolipids [32,33]. The absence of DGDG might reduce the stability of the membrane bilayers, the activity of membrane bound proteins and the membrane permeability [34,35]. Thus, MGDG and DGDG play important roles in the structural stabilization and function of membranes, and are fundamental in the trafficking of lipids between subcellular compartments [36].
As mentioned, PF2 contained novel acidic glycophosphatidyl glycerols (GL4-GL7). Phosphatidyl glycerol (PG) plays a critical role in the function of photosystem II (PSII) in the thylakoid membrane and is believed to be the only lipid completely essential for oxygenic photosynthesis, with other plastidic lipids such as galactolipids MGDG and DGDG, found to be replaceable with glucolipids, or in the case of SQDG, dispensable [37,38]. Increased PG under salt stress was observed in epidermal bladder cells of the halophyte M. crystallinum, and in salt-tolerant buffalo grass [39,40]. As an integral component of photosynthetic membranes it was speculated that the salt-induced increase in PG levels is important for maintaining the functioning of PSII by protecting the photosynthetic apparatus and stabilizing photosynthetic processes, such as ATP synthesis and light-harvesting complex II (LHCII). In fact, ultrastructural damage of thylakoid membranes has been observed with the decline in PG in leaves from salt-stressed Sulla carnosa and S. coronaria [41]. PG is therefore a vital lipid, mainly for its role as a cofactor of photosystems and SQDG appears as a sort of stand-in actor, playing parts of PG functions when phosphate is limited. To our knowledge glycophosphatidyl glycerols (GL4-GL7) are new natural products produced in a halophyte.
It is noteworthy, that saturated C14 and C16 fatty acids are constituents in both the acidic glycerolipids. Acetic acid was also found to be the constituent of diphosphatidyl glycerol (GL7, cardiolipin). Cardiolipin is a unique phospholipid which is almost exclusively located in the inner mitochondrial membrane when it is biosynthesized and is essential for the optimal function of numerous enzymes that are involved in mitochondrial energy metabolism [42].
Saturated lipids generate liquid order phases, and unsaturated lipids generate liquid-disordered phases, thereby the presence of fatty acids and their state of saturation can directly affect membrane fluidity [43]. Thus, reduced lipid unsaturation of fatty acids was assumed to be correlated with a decrease in membrane fluidity and it was thought that this would limit the uptake of Na+ and Cl− across the plasma membrane by directly regulating transporters involved [44]. A reduction in membrane fluidity is also thought to be necessary to prevent leakage of the compatible solute glycerol, out of the cell and diffusion of potentially harmful ions into the cell, helping to maintain the cell osmotic pressure balance between the internal and external environments [45]. Furthermore, a reduction in the degree of unsaturation of lipid fatty acids is related to a decrease in the susceptibility of the membrane to oxidative damage, helping to protect membrane integrity [46]. The saturation of acidic lipids may be a consequence of high light intensity as observed by in three species of macroalgae (Ulva pertusa, Grateloupia sparsa and Sargassum piluliferum) or enhancement of lipoxygenase activity under stress as observed in S [47] portulacastrum under cadmium stress. Lipoxygenase can efficiently start the peroxidative breakdown of both polyunsaturated free fatty acids and complex lipids, and/or generate reactive oxygen species that can increase the deterioration and permeability of membranes [48]. However, Kannan reports an increase in the antioxidant enzymes polyphenol oxidase (PPO), superoxide dismutase (SOD) and catalase (CAT) under salinity in the leaves of S. portulacastrum L [49]. The elevation of saturated fatty acids in most glycerolipids under the acclimation to pre-monsoon abiotic stresses suggests that the activity of specific types of lipolytic enzymes increases during summer. This species may have an efficient strategy which can be related to anti-oxidative production against oxidative stress.
Polyols or polyhydric alcohols are among the compatible solutes involved in osmoregulation and are thought to accumulate in the cytoplasm of some halophytes to overcome osmotic disturbances caused by high concentrations of inorganic ions compartmentalized in vacuoles. In addition to their role in osmoregulation, polyols also function as oxygen radical scavenger thereby protecting the proteins from oxidative damage in drought stressed plants [50,51]. Glycerolipids particularly glycophosphatidyl glycerols are polyols and one can easily understand their role in osmoregulation by replacing hydroxyl proton under high salinity and their oxygen radical scavenger property protecting the plant against oxidative damage. The protection against oxidative damage is further enhanced by flavonoids constituents of the plant which are well known antioxidants.
Lipid profiling provided new information about the identities of the polar lipid molecular species produced during abiotic stresses. It was thus evident that environmental conditions have significant impact on lipid metabolism in the halophyte. Under abiotic stress the halophyte produces acidic phospholipids, phosphatidyl glycerols and sulfonolipids. S. portulacastrum is an obligate halophyte with unaffected growth at 100-200 mM NaCl in the aqueous medium and could still grow and survive up to 1M NaCl. The emerging data on adaptability of the plant exposed to various abiotic factors revealed that the halophyte species maintains its growth by sequestration of Na+ ions into the vacuoles to maintain the osmotic balance between vacuole and cytoplasm. The growth in stressful hyper saline conditions requires that cells accumulate osmoprotectants. In many instances, these are polyhydric alcohols. In this report we have observed accumulation of novel glycerophospholipids (GL3-GL7)/polyols with saturated fatty acid composition, under abiotic stress conditions prevailing during the pre-monsoon season. These polyols accommodates sodium ions by substituting hydroxyl protons and being oxygen radical scavengers, would protect the plant against oxidative damage and their saturated fatty acid composition contributing to the homeostasis of membrane fluidity and permeability.
Data acquisition of glycerolipids samples using ESI-MS/ MS
Mass spectra were recorded, in the positive ion mode, on a QTOFXL MS/MS Applied Biosystem instrument equipped with MDS Sciex Analyst Software (Concord Ontario, Canada). In addition to full scan mass spectra, collision induced dissociation (CID) was undertaken in the MS/MS mode to yield diagnostic product ion mass spectra, which were characteristics of the structural moieties present in the analyte. While infusing the sample, the collision energy was varied between 20–50V so as to obtain optimum product ion mass spectra. The sample after dilution with methanol (MeOH) was directly infused at a constant flow rate of 10μl/ min. into the ion spray source using an integrated syringe pump. Nitrogen was used as nebulizing and desolvation gas.
Collection of biological material
Plant of S. portulacastrum (shoots) were collected from the location at Sinquerim beach, Goa, India (15.4991ºN, 73.7675ºE) during pre-monsoon (March, 2017) and post- monsoon (September, 2017) periods. The shoot samples were deposited at National Institute of Oceanography Repository and Taxonomic Reference Centre (NIOTRC 0442), Dona Paula, Goa, India.
Pre-monsoon season in Goa is characterized by high temperature (≥35ºC) and salinity (33.3ppt) coupled with high humidity (75%). On the other hand lower temperature (28-30ºC), salinity (21.5ppt) and moderate humidity are characteristics of post-monsoon period.
Extraction and isolation of glycolipids
Each sample (3kg, dry weight of shoots of whole plant) of sea purslane was thoroughly cleaned and extracted thrice in MeOH at room temperature (27°C), each extraction lasting three days. The combined MeOH extract was concentrated in vacuum at 40°C to reduce the volume to 200 mL. This was followed by the partitioning of the concentrated mixture successively with chloroform and n-butanol. The butanol fraction (113g) was filtered through Sephadex LH20 (25-100 mm) (Pharmacia, Sweden) and eluted using methanol (500 mL). Sub-fractions of 20 mL each were collected separately in test tubes and analysed by TLC (Merck, aluminium–backed sheets, silica gel 60 F254). Spots were developed in a solvent system comprising of petroleum ether: ethyl acetate (1:1, v/v). The plates were air dried and sprayed with 5% methanolic sulfuric acid to visualize the spots when heated in an oven at 100°C. Fractions yielding purplish pink spots were combined and subjected to silica gel column chromatographic purification to give the post-monsoon glycerolipid fraction (PF1) (Rf =0.42, yield: 63 mg). Repeated chromatographic separation with increasing concentration of ethyl acetate in petroleum ether of fraction PF1 yielded 58.5 mg of pure GL1.
For pre-monsoon collection the same methodology was used to obtain PF2 (yield: 28.9 mg) and 26.2 mg of GL1.
Acid hydrolysis of glycerolipids (PF1–2)
To establish the nature of sugar constituent and its configuration, purified glycolipids PF1and PF2 fractions (4-8 mg) were separately subjected to acid hydrolysis in methanol by following the procedure reported by [52]. TLC comparison with standard sugars and agreement of its optical rotation (+150°) with literature values (+150.7°) (Takahashi and Ono, 1973)) identified the carbohydrate constituent of glycolipid as α-D-galactose. Corresponding D-galactose with beta configuration has optical rotation of (+) 52.8° [53].
The work was supported by Council of Scientific and Industrial Research (CSIR) under the (OLP 2006).
Solimabi Wahidullah, Safia Khan and Prabha Devi contributed to the experimental work in the laboratory, interpretation of data and preparation of the manuscript.
The authors declare no conflict of interest.
We thank Dr Sayeeda Wafar for identifying the mangrove. We also sincerely thank Director, National Institute of Oceanography, (CSIR), for his keen interest in the work. This manuscript has NIO contribution No.
Citation: Wahidullah S, Khan S, Devi P (2021) ESI-MS/MS Characterization of Glycerolipids and Seasonal Alteration in their Composition in Sesuvium Portulacastrum (Linnaeus) -A Salt Marsh Halophyte. J Glycobiol 10:180
Received: 08-Nov-2021 Accepted: 23-Nov-2021 Published: 30-Nov-2021 , DOI: 10.35248/2168-958X.21.10.180
Copyright: © 2021 Wahidullah S, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.