Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Research Article - (2020)Volume 11, Issue 1
Comparison of various technologies for uranium recovery from sulphuric acid leach solutions shows that the moving bed systems are more effective processing routes than fixed bed. Developed system using air pulsation for resin moving was tested and examined for uranium recovery from Gattar pilot plant project (North Eastern Desert, Egypt) sulphuric acid leach liquor using Chinese resin D263B. Recovery equilibrium and kinetic isotherms are established. The obtained results showed high performance and the validity of derived pulsed resin column for uranium extraction. Elution behavior was improved by using intense fractional eluent which gives efficient results.
Moving bed; Developed system; Air pulsation; Equilibrium isotherm; Kinetics
In the earliest uranium plants, concentration and purification of solutions was achieved by selective adsorption of uranium by an ion exchange resin in fixed-bed columns. The development of the continuous ion exchange system has been a big breakthrough in uranium extraction technology. The fluidized bed technique has been studied and applied in uranium industry from 1970 and now research and application of the densely packed-moving bed techniques in uranium industry are in progress [1]. Problems due to a decrease in particles mean radius during adsorption prompted us to use a fluidized bed. It is also possible with this reactor to treat solutions containing suspended solids which would clog fixed beds. In the same time during elution, the addition of the less dense eluant caused the resin bed to sink slowly as the dense feed solution was displaced eluant flowed down through the column, which was operated as a fixed bed. The elution characteristics, after the bed settled as a plug to the bottom of the column, were the same as observed for a fixed bed. [2,5]. The decrease in particle radius (or increase in apparent density of the resin) produces a contraction of the fluidized bed: unloaded particles remain at the top of the bed and a density gradient appears throughout the column, leading to a stabilization of the fluidized bed [3,4].
An application in a fluidized bed reactor requires a good understanding of equilibrium and kinetics properties of the adsorption. In the perspective, equilibrium relationships, generally known as adsorption isotherms, describe how pollutants interact with the adsorbent materials, and thus are critical for optimization of the adsorption mechanism pathways. Namely; the surface properties, capacities of adsorbents, and effective design of the adsorption systems [4,5]. In general, an adsorption isotherm is an invaluable curve describing the phenomenon governing the retention (or release) or mobility of a substance from the aqueous porous media or aquatic environments to a solid-phase at a constant temperature and pH [6,7]. Adsorption equilibrium (the ratio between the adsorbed amount with the remaining in the solution) is established when an adsorbate containing phase has been contacted with the adsorbent for sufficient time, with its adsorbate concentration in the bulk solution is in a dynamic balance with the interface concentration [8,9].
Developed system theory depends on acceleration of resin adsorption and elution rate through the expansion of the ion exchange bed area (EBA), using pulsed air. In EBA, adsorbent particles with defined size and density distribution are fluidized by a mobile phase directed upwards from 'classified' fluidized bed which commonly termed an expanded bed as in the following diagrams [10-12]. Central to the performance of the EBA system is that the axial mixing is low and the void fraction is increased which allows the application of the un-clarified liquors. When the bed was pulsed instead of agitated, solids was passed equally as well [13,14].
For the special case in which the feed solution had a higher density than the resin, the bed floated and expanded even when subjected to a downward flow; this action allowed solids to pass through the bed. The absorption characteristics of the expanded bed approached those of a fixed bed. Although the bed readily passed the solids, the bottom screen, used to retain the resin, plugged with particles of resins and solids that were gradually carried down [15]. This difficulty was overcome by pulsing the column so that liquid passed back and forth through the bottom perforated plate and forced the fine solids through the plate. The pulses were generated by cyclic variations of the air pressure to the waste outlet weir.
The present study is concerned with developing the operating system (fixed bed ion exchange column) in Gattar uranium pilot plant project which constructed at 2001 year near Gebel Gattar located in the northern part of the Eastern Desert of Egypt. The developed system depends on fluidizing the resin bed more quick through stream solution using air pulsing technique. However, this was eventually dropped in favor of a contactor that would be hybrid among, resin in fluidized pulsed contactor, suspended bed column and a multistage column (Figure 1).
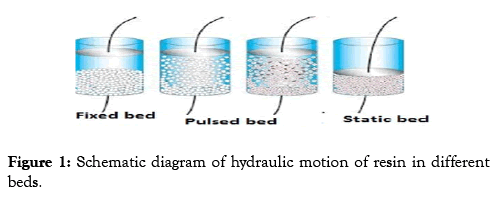
Figure 1: Schematic diagram of hydraulic motion of resin in different beds.
The operated ion exchange (D263B) is used to study the relevant adsorption factors under developed system including the following: (a) laboratory tests to determine the optimum operating conditions to give the best performance of resin on new operating system, (b) adsorption isotherm in specific leach liquor/pulsed bed and rate of adsorption, and (c) elution isotherm, using different eluents and rate of elution.
According to the used resin is in chloride form, it was necessary to convert of the resin from chloride form to sulfate form is accomplished in column operation by contacting the resin with 3% H2SO4 plus 10% Na2SO4 solution until there is almost no Cl- in the effluent, the retention time is 10 minutes followed by washing resin by fresh water. Resin capacity was determined by direct contact of a certain amount of resin (5 ml wet settled resin (wsr) with synthetic 1 gmU/l) under optimum conditions: L/R ratio=100, 2 hr contact time, 250 rpm agitation speed, 30°C room temperature and 1.7 pH. This experiment gives 66 mgU/mlR (wsr) as a maximum capacity of the studied resin. Based on the earlier experiments, a bench test was carried out with 100~300 ml volume of wetted D263B resin (of 0.6 g/ml density, 0.55 mm particle size, pore volume 40%, wet density 1.06-1.11 g/ml and moisture content 50%) packed in a glass column.
The developed column consists of glass tube of 5 cm internal diameter and its active section was 30 cm and backed with approximately certain amount of resin and separated by 60 μm aperture wedge-wire screens which held the resin in each stage. The column was supplied with a motive solution pump sourced fluid from the base of the column and pulsed air generator as illustrated in Figure 2.
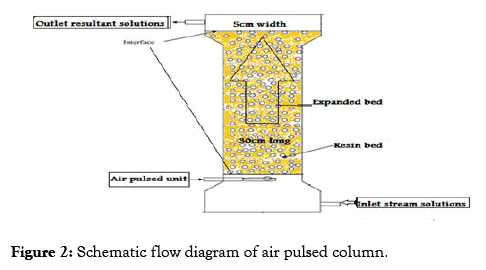
Figure 2: Schematic flow diagram of air pulsed column.
The uranium concentrations were determined spectrophotometrically using a spectrophotometer (Shimadzu UV-120-02) with Arsenazo III [16]. All reagents used were of analytical grade and their respective solutions were prepared with distilled water.
Optimization of the adsorption relevant factors
Mass transfer performance in a pulsed extraction column with 50 mm diameter and 350 mm long affected by four factors: pulsed air flow rate, frequency (pulses number per minute) and PLS flow rate. The flow rate volumes were expressed in Bed volume (Bv) which refers to the volume of solution or air equivalent to the resin volume in place.
Effect of contact time in batch equilibrium: Normally, good resin not only has a large capacity, but also has fast adsorption speed. In practice, such a resin has lower inventory with high operation performance. This effect was studied under the following conditions: 10 ml of (wsr) in 1000 ml PLS (500 ppm), pH=1.75 and 250 rpm at different contact times.
Figure 3 shows that the adsorption speed is very fast at the first 3 minutes then becomes slow; this suggests that during the first 4 minutes, the adsorption mechanism is controlled by particle diffusion, then, it became film diffusion controlled. Thus, 5 (or 6) minutes are the required contact time to reach equilibrium which gives extraction efficiency 97%. Accordingly, a fluidized bed will be benefit to the resin adsorption. Thus, 5 min. is the contact time required to reach equilibrium at which the uranium attained 49 mgU/mlR.
Figure 3: Effect of contact time on uranium extraction efficiency %.
Effect of pulsed air flow rate: Figure 4 illustrates the effect of pulsed air flow rates under fixed conditions (100 ml resin, L/ R=100, U=500 ppm, pH=1.75, 50 puls/min. and PLS flow rate of 8 BV/hr) and shows that as the compressed air flow rate increase the extraction efficiency increases until reaching its maximum extraction efficiency of 95% equivalent to 47.5 mgU/mlR at the pulsed air bed volume equal 6 with bed expansion ratio 1.98. It is noted that the expanded bed was increased periodically with pulsed air and consequently the extraction efficiency percent. This can be attributed to the larger surface area for ion exchange process to be carried out by the pulsed air.
Figure 4: (a) Effect of pulsed air flow rate on uranium extraction efficiency % and (b) on bed expansion ratio H/H0.
Effect of pulsed air frequency: Increasing number of pulses per minute during the batch extraction experiments and fixed other conditions gives less effect on the extraction efficiency at high values from 30-60 pulse/min. At low number of pulses (10-30 pulse/min) (Figure 5), there are large bubbles formed that may make back adsorption and flooding this consequently decreases the ion exchange rate.
Figure 5: Effect of frequency on uranium extraction efficiency%.
Effect of pregnant leach solution (PLS) flow rate: To evaluate good mass transfer performance of uranium extraction in pulsed column, the PLS flow rate was studied under the obtained extraction optimum conditions with different PLS flow rates. The resulted effluents will be analyzed for uranium each 10 Bv. The adsorption performance of the resin for service in column was presented by means of adsorption or loading curves as shown in Figure 6. These curves describe the breakthrough profiles for uranium at different flow rates in which uranium concentration of the column effluent (mg/l) is plotted against cumulative bed volumes. All curves show slightly a sharp profile which indicates the relatively high affinity of this resin with the metal. Although the profiles seem similar, Bv corresponding to the breakthrough point is slightly different.
Figure 6: Breakthrough profiles for different flow rates.
Figure 6 shows that the breakthrough point was reached at approximately 120 Bv for flow rate of 6 and 8 Bv/hr while, for the flow rate of 10 and 12 Bv/hr the breakthrough point was reached at about 80 Bv. Therefore, the recommended operation flow rate should be 8 Bv/hr which corresponds to the retention time of 4 min. For PLS flow at 6 and 8 Bv the resin reach maximum capacity at 140 Bv, while at flow rates 12 and 14 Bv resin saturation not reached after passing larger PLS volume.
Adsorption isotherms: Adsorption isotherms were generated for the adsorption of uranium onto the strong-base anion exchange resin, in the sulphate form, by contacting the resin and PLS until saturation reached under the obtained operating optimum conditions as illustrated in Figure 6. McCabe-Thiele construction was done using the adsorption isotherm generated and illustrated in Figure 7. The Langmuir equilibrium isotherm is almost fit with equilibrium adsorption data.
Figure 7: Uranium adsorption isotherm.
Uranium adsorption isotherm and kinetics of uranium uptake are presented in Figures 7 and 8. Adsorption isotherms illustrated shows the sharpness curves which indicate good adsorption performance in the air pulsed column.
Figure 8: Rate of uranium adsorption.
Optimization of elution relevant factors
1M sodium chloride acidified by 0.1M H2SO4 was used as an eluent solution in Gattar pilot plant as its advantage of low cost and plentiful resource. As in the adsorption process the similar procedures would be repeated but as in the elution manner.
Effect of contact time: The elution kinetics will determine the required time to reach equilibrium during the elution process of ion exchange resins which was investigated to determine the minimum residence time required to attain equilibrium. Figure 9, shows that the equilibrium elution was reached at 8 minutes.
Figure 9: Effect of contact time on Uranium elution efficiency %.
Effect of air pulsed flow rate: The achieved experiments show that there is a considerable effect of pulsed air flow rate on elution efficiency, elution equilibrium was reached at 8 Bv/min flow pulsed air.
Effect of pulsation frequency: Experiments illustrated in Figure 10 shows that the elution efficiency increases sharply with pulses increases until reach 50 pulses/min the equilibrium elution is reached. The sharp increase could be attributed to that frequency increase the resin grain speed which consequently increases the ion exchange process (Figure 11).
Figure 10: Effect of pulsed air flow rate on uranium elution efficiency%
Figure 11: Effect of pulsed air frequency on Uranium elution efficiency %.
Effect of eluent flow rate: Assumptions based on equilibrium and kinetics of the elution was confirmed through column elution testes, where elution was performed at different flow rates.
The obtained data shows that there is a minimal difference between the elution efficiency observed within the flow rates evaluated from 1 to 4 Bv/hr, with almost all flow rates achieving 27% uranium elution. When eluent flow rate increases than 4 Bv/hr elution efficiency decreases sharply this attributed to less contact time operated. To get accurate good elution performance, elution kinetics of column was tested at eluent flow rate from 2, 3 and 4 Bv/hr which showed little difference in elution efficiency. Consequently, as in Figure 12 elution curves are relatively same indicating very little difference in elution performance for three flow rates and good elution performance.
Figure 12: Effect of eluent flow rate on uranium elution efficiency %.
Elution isotherm: The most commonly used eluent agents are chloride, nitrate, dilute sulphuric acid and carbonate. When chloride and nitrate is used to eluate uranium from loaded resin, the resin is converted to Cl or NO3 form. After elution, resin is transferred back to the adsorption phase and in the next adsorption, the Cl- or NO3 will be passed back into barren solution. When sulphuric acid and sulphate is used as eluent to elute uranium from loaded resin, the adsorbed solution can be recycled totally to the leach process. In this process the loaded resin is contacted with different eluents [8,16].
It can be seen from Figure 13 that by using the intense fractional process (1M H2SO4+1.5M Na2SO4), the uranium elution peak concentration is higher and the pregnant liquor volume is less than that of the conventional elution process which proved their best efficiency. Since the average total Bv for intense fractional and conventional elution processes were 14 Bv, 16 Bv and 18 Bv respectively, where the elution efficiency were 95.6, 93.2 and 91.24% respectively (Figure 14).
Figure 13: Break through profile for uranium elution with different eluent reagents in air pulsed column.
Figure 14: Equilibrium isotherm and kinetics of uranium elution by different eluent in Air pulsed column.
Equilibrium elution isotherms and rate of elution indicated that uranium elution by different eluants have an equilibrium constraint (Figure 14). For effective uranium elution, a specific volume of eluant was required. Results indicated that the rate of elution would be less of a determining factor on the elution efficiency (with relatively long elution resin residence times) achieving almost complete uranium elution after 3 hrs.
The performed batch experiments demonstrate adsorption and elution results correlated reasonably well with the predictions that were made from the laboratory test work. Moreover, these results confirmed the improved performance of developed system under the optimized operating conditions. The developed system raise the PLS flow rate up to 8 Bv and 4 Bv/hr using power of 6 Bv and 8 Bv/min. of compressed air pulsed with 50 pulse/min., respectively during adsorption and elution process respectively. Intense fractional eluent (1M H2SO4+1.5M Na2SO4) give more efficient results than the operated one since the elution efficiency reached to 97%.
Citation: Morsy WM (2020) Enhancing the Uranium Recovery Performance in Gattar Pilot Plant Using Pulsed Column Technique. J Chem Eng Process Technol 11:340. doi: 10.35248/2157-7048.20.11.400
Received: 27-Jan-2020 Accepted: 17-Feb-2020 Published: 24-Feb-2020 , DOI: 10.35248/2157-7048.20.11.400
Copyright: © 2020 Morsy WM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.