
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2021)Volume 11, Issue 4
In order to determine whether clinoptilolite, a naturally occurring zeolite, had any toxic effect on keratinocyte cells when combined with lead (II) acetate, real time cell analysis and MTS assays were performed. Herein, Micronized Zeolite (MZ) was obtained by tribomechanical micronization of natural clinoptilolite. The effect of lead (II) acetate (Pb (CH3COO) 2.3H2O) adsorption of clinoptilolite at different durations (30 minutes, 1 hour, 2 hours, 4 hours, 8 hours) was investigated. When 5% clinoptilolite was applied to the lead (II) acetate solution, the adsorption capacity was 55.15% in 30 minutes, 58.09% in 1 hour, 71.62% in 2 hours, 82.66% in 4 hours, 87% in 8 hours. These results were in parallel with cell culture studies which showed when lead is treated with tribologically micronized zeolite (Clinoptilolite) it increases the cell viability.
Clinoptilolite, had an adsorbing effect on lead (II) acetate, did not show toxic effect on keratinocyte cells, and even eliminated lead (II) acetate toxicity. It might suggest an alternative natural cell protecting chelator.
Lead (II) acetate; Clinoptilolite; Ethylene diamine tetra acetic acid; Keratinocyte cell; Cytotoxicity
Humans are exposed to hazardous heavy metals via inhalation and ingestion. These deleterious compounds lead to oxidative stress in cells let it them to formation Reactive Oxygen Species (ROS) [1,2]. One of the most abundant toxic heavy metals is lead that tend to accumulate in soft tissues in the body, causing numerous diseases and disorders according to exposure, dose, duration and chemical state of lead [3]. Even if small doses of this element may cause hormone disruption [4]. Lead exposure can be classified into two groups; acute and chronic. Chronic lead exposure is a result of environmental pollution. It affects many organ systems such as nervous, reproductive, hematopoietic system [5]. Besides it may result in skin disorders and including sensitive skin, premature skin aging and skin dryness. It penetrates the skin barrier and may cause inflammation damages by reacting with skin proteins, lipids and DNA molecules [6]. Long Hwang et al. were investigated cytotoxicity of HgCl2 using HaCaT cell line [7]. Similar study was done by Soon Bae and colleagues to show cytotoxic profile of Arsenic, Cadmium, Chromium, and Lead on Human Keratinocytes in a dose dependent manner [8].
Ethylene Diamine Tetra Acetic Acid (EDTA) is the most common chelating agent used worldwide. Ethylene Diamine Tetra Acetic Acid (EDTA), which is in the structure of aminocarboxylic acid, reacts with multivalent metal ions and forms complex compounds to inactivate metal ions. It is widely used in industrial applications, canned foods and home care products [9]. Biodegradation of Ethylene Diamine Tetra Acetic Acid (EDTA) in the nature is very low [10]. In addition, studies have reported that (EDTA) leads to cellular toxicity [11]. Showed that even in low levels of Ethylene Diamine Tetra Acetic Acid (EDTA) leads to cellular death in cultured rat kidney cells [12]. Demonstrated that (EDTA) cause toxic effect on corneal and conjunctival epithelial cells [13].
Zeolites are a type of aluminosilicate minerals that include natural and synthetic species with microporous structure that is formed from cages. This architecture provides it remove heavy metal via strong ion exchange capacity. Because of their high Cation Exchange Capacity (CEC) it is used in environmental, agricultural, industrial, and biological technologies [14]. The effectiveness of cation exchange is directly related to the particle size of the zeolite [15]. Natural zeolites such as clinoptilolite is a non-toxic zeolite that has strong adsorptive and ion exchange capacity [16]. Clinoptilolite selectively removes lead (Pb2+), zinc (Zn2+), nickel (Ni2+), cadmium (Cd2+), iron (Fe2+), copper (Cu2+), and manganese (Mn2+). Natural clinoptilolite has antiviral anti-cancer and anti-oxidative effects [16-20].
Based on these, we assumed that the clinoptilolite pretend to chelating agent and could be eliminate the toxic effect of lead (II) acetate on keratinocytes in a time dependent manner.
Materials
Lead (II) acetate trihydrate, 467863, SIGMA. Lead Test, 109717, Merck (contains Potassium cyanide solution, hydroxylammonium chloride - ammonia solution), Spectrophotometer, Agilent Cary 60 UV/VIS, 84% clinoptilite<10-micron zeolite micronized with a tribomecanic micronisation system, DYNAMICROTECH GmbH. MTS Cell Proliferation Colorimetric Assay Kit, Biovision. xCELLigence Real Time Cell Analysis (RTCA), ACEA Biosciences.
Preparation of lead (II) acetate-%5 zeolite complex in different time points
In this study, Pb (CH3COO) 2.3H2O (Merck) was used to study the lead (II) acetate adsorption of micronized zeolite. 3.41 g/l Pb (CH3COO) 2.3H2O metal ion solution was prepared in the work. In the micronized Zeolite treatment, 5 g zeolite/100 ml metal ion solution, 1 gram (EDTA)/100 ml metal ion solution and 0.1 gram (EDTA)/100 ml metal ion solution were mixed at 200 rpm for different times (30 minutes, 1, 2, 4, 6, 8 and 24 hours) at atmospheric conditions in a shaking incubator at 24°C. At the end of the application zeolite was precipitated by centrifugation at 9000 rpm for 10 minutes and the supernatant fraction was taken and prepared for lead and cell culture assays.
UV/VIS spectrophotometer analysis of lead (II) acetate
Before starting experiment, test solution was filtered and then 1000 times diluted with deionized water in a 100 mL calibrated flask. 0.50 ml potassium cyanide solution was pipetted into a test tube. 0.50 ml hydroxylammonium chloride-ammonia solution was added and the tube was mixed. 8.0 ml pretreated sample (1000 times diluted with water) was added and the tube mixed with vortex for 60 seconds. The measurement solution was filled into the 10 mm cell. The absorbance was measured at 525 nm against a corresponding reagent blank.
Calculation
μM Pb × 2.64=Absorbance × 6.25 × 1.83 × 1000 6.25: coefficient of the analysis method (Merck 109717 Lead Test) 1.83: coefficient of molecular weight Pb/Pb(CH3COO)2.3H2O 1000: dilution fold 2.64:μM/ppm conversion.
Cell culture
The HaCaT cells were cultured in DMEM medium with glutamine containing 10% FBS, 10 IU/ml of penicillin and 10 μg/ml of streptomycin. The cells were cultured at 37°C in a 5% CO2 incubator. Cells were passaged twice a week.
Cell viability assay
HaCaT cells were seeded into 96-well plates at a density of 1×104 per well with 100 μl medium and treated with the following: Clinoptilolite (2,5% and 5%), lead (II) acetate (2000 and 4500 μM), lead (II) acetate-5%-Clinoptilolite (2000 and 4500 μM) with different time points (30 min, 1 hour, 2 hour, 4 hour, 8 hour), (EDTA) (2.5% and 5%), lead (II) acetate-5% (EDTA) (2000 and 4500 μM). Wells with substance free medium were used as negative control. After 24 hours, 10 μl of MTS reagent was added to each well and the plates were incubated for 4 hours at 37°C. The amounts of formazan in the wells were read on a microplate reader at 490 nm.
Real time cell analysis
Human Epidermal Keratinocyte (HaCaT) cell line was expanded in DMEM, 10% FBS and subjected to tissue culture E-Plate that was used to generate dynamic real-time data with measuring electrical impedance across interdigitated micro-electrodes on the bottom of the plate. Clinoptilolite (5%), lead (II) acetate (2000 and 4500 μM), lead (II) acetate-5% clinoptilolite (2000 and 4500 μM, 1 hour and 8 hour) were prepared and applied to the E-plate. The results were followed at real time by the xCelligence system.
Morphological changes
HaCaT ells were seeded into 96 well plates (1×104 cells/well) in 100 μl medium and let incubation for 24 hours. Cells were treated with lead (II) acetate (2000 and 4500 μM), lead (II) acetate -5% Clinoptilolite (2000 and 4500 μM, 8 hour), and lead (II) acetate-5% (EDTA) (2000 and 4500 μM, 1 hour) After 24 hours incubation, the medium was replaced with fresh one. Cells were observed under inverted microscope.
Statistical analysis
All of the test materials were studied triplicate. Student’s t test was used to assess the relationships between variables. Statistical significance was defined as P values of <0.05.
UV/VIS spectrophotometer analysis of lead
As shown in Figure 1, the absorbance was linear for 450-9000 μM of lead at 525 nm (R2=0,99). In the case of continuous clinoptilolite adsorption, the amount of lead in 3410 ppm lead acetate ionic solution of 5% clinoptilolite was 55.15% at 30 minutes, 58.09% at 1 hour, 71.62% at 2 hours, 82.66% 87.95% in the 8th hour (Figure 2). Statistically, there was a significant difference between 2nd hour and 4th hour while there was no significant difference between 30 minutes and 1 hour. The time that lead acetate is removed most is the 8th hour. However, the lead treated with 5% (EDTA) adsorbed almost all of the lead acetate in 1st hour.
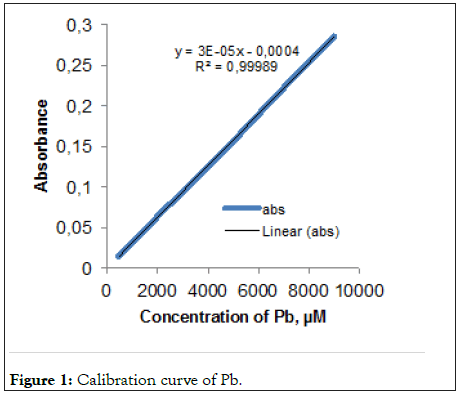
Figure 1: Calibration curve of Pb.
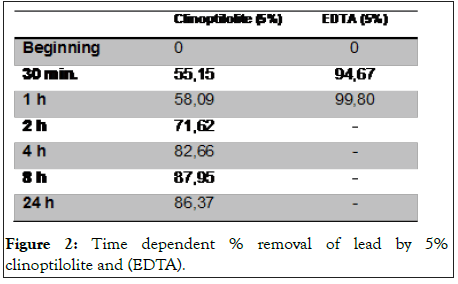
Figure 2: Time dependent % removal of lead by 5% clinoptilolite and (EDTA).
Cell survival effects of HaCaT cells treated with lead (II) acetate, lead (II) acetate -5% Zeolite and lead (II) acetate -5% (EDTA) at 2000, 4500 μM concentrations
Comparison of % cell survival effects in HaCaT cells treated with lead (II) acetate, lead (II) acetate-5% clinoptilolite at different time points and lead (II) acetate-5% (EDTA) at 1 hour (2000 and 4500 μM) is shown in Figure 3. The cell survival effects were 48% and 22% when the keratinocytes were treated with 2000 μM and 4500 μM of lead (II) acetate, respectively. It was observed that lead (II) acetate was toxic alone but when treated with 5% clinoptilolite for different times cell viability of keratinocyte cells were increased depending on treated times. Also, Ethylene Diamine Tetra Acetic Acid (EDTA) was used as a positive control with known strong chelating properties. Lead (II) acetate was treated with 5% Ethylene Diamine Tetra Acetic Acid (EDTA) in 1 hour. As shown in Figure 3, Ethylene Diamine Tetraacetic Acid (EDTA) has significantly reduced cell viability at both concentrations (2000 and 4500 μM) despite being treated with lead for 1 hour and 24 hours. In Figure 4, cells treated with clinoptilolite showed a growth above the control whereas cells treated with Ethylene Diamine Tetra Acetic Acid (EDTA) showed toxic effect in a dose dependent manner.
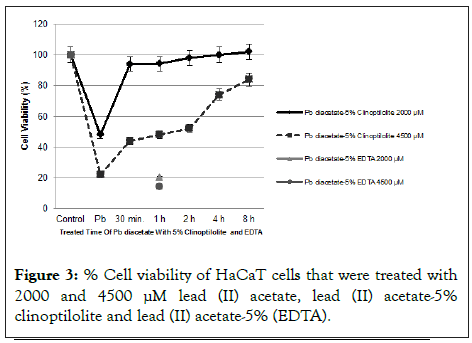
Figure 3: % Cell viability of HaCaT cells that were treated with 2000 and 4500 µM lead (II) acetate, lead (II) acetate-5% clinoptilolite and lead (II) acetate-5% (EDTA).
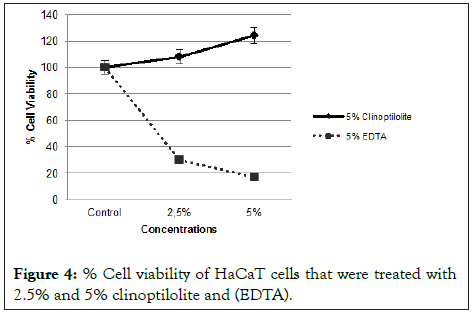
Figure 4: % Cell viability of HaCaT cells that were treated with 2.5% and 5% clinoptilolite and (EDTA).
Cell viability assay was confirmed with Real Time Cell Analysis (RTCA) system that measures impedance-based signals and provides dynamic information. The cellular response was continually monitored for 66 h. Red line indicates the untreated negative control cells. As shown in Figure 5, lead (II) acetate at 2000 (pink) and 4500 μM (black) concentrations were decreased cell viability. Blue and brown colour indicates lead (II) acetate-5% Clinoptilolite (2000 μM, 1 hour) and lead (II) acetate-5% clinoptilolite (4500 μM, 1 hour), respectively. Cyan and green colour indicates lead (II) acetate-5% Clinoptilolite (2000 μM, 8 hour) and lead (II) acetate-5% Clinoptilolite (4500 μM, 8 hour) respectively. It has been observed that as the time during which the clinoptilolite is treated with lead diacetate increases the cell viability. Also, cells treated with clinoptilolite showed a growth above the control.
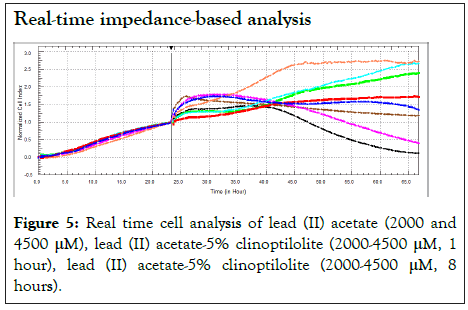
Figure 5: Real time cell analysis of lead (II) acetate (2000 and 4500 µM), lead (II) acetate-5% clinoptilolite (2000-4500 µM, 1 hour), lead (II) acetate-5% clinoptilolite (2000-4500 µM, 8 hours).
Effect of lead (II) acetate and lead (II) acetate-5% Clinoptilolite on HaCaT cell morphology
Keratinocytes were treated with lead (II) acetate, lead (II) acetate-5% clinoptilolite for 8 hours, lead (II) acetate-5% (EDTA) for 1 hour or left untreated. According to the Figure 6, morphology of lead (II) acetate and lead (II) acetate-%5 (EDTA) treated cells (2000 and 4500 μM) was changed features like shrinking, blebbing whereas lead (II) acetate-%5 clinoptilolite treated cells was normal. Cells were observed under inverted microscope.
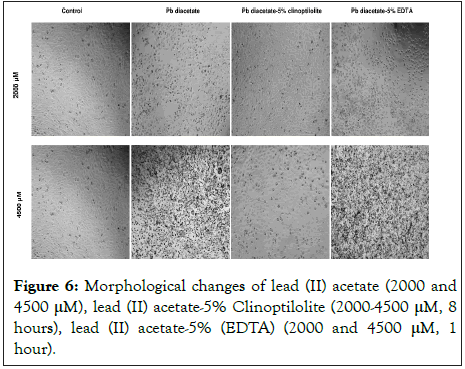
Figure 6:Morphological changes of lead (II) acetate (2000 and 4500 μM), lead (II) acetate-5% Clinoptilolite (2000-4500 μM, 8 hours), lead (II) acetate-5% (EDTA) (2000 and 4500 μM, 1 hour).
Ethylene Diamine Tetra Acetic Acid (EDTA) is one of the bestknown chelating agents for heavy metal ion removal. Besides this feature, it has a toxic characteristic for cells [12,13]. In this work, we compared the lead (II) acetate adsorption properties of clinoptilolite and Ethylene Diamine Tetra Acetic Acid (EDTA). Lead (II) acetate adsorption capacity of clinoptilolite reached saturation at the end of the 8th hour and the concentration of lead (II) acetate in the environment was stabilized. It is considered that if the clinoptilolite concentration is increased, the lead (II) acetate stabilization period is shortened and the amount of lead (II) acetate that is released in the environment will reach a lower value or completely adsorbed. From the obtained results, it can be concluded that clinoptilolite is effective in the adsorption of lead (II) acetate. In our study, clinoptilolite adsorbed 87.95% of lead (II) acetate at the 8th hour [21]. Found that nearly 100% of clinoptilitis was adsorbed in a very short period of time. This is thought to be due to the different amount of working solution and clinoptilitis [22]. Reported that the adsorption of zeolite of Pb was 88.99% at 40 mg/L. This result is similar to the results of our work. 5% Ethylene Diamine Tetra Acetic Acid (EDTA) adsorbs almost all of the lead (II) acetate in the first hour however clinoptilolite at the same concentration adsorbs 58,09%. When these studies were carried out in cell culture, clinoptilolite supported cell viability of keratinocyte cells while Ethylene Diamine Tetra Acetic Acid (EDTA) showed toxic effect. % cell survival effects of lead (II) acetate and 5% clinoptilolite treated lead (II) acetate at 30 min, 1st hour, 2nd hour, 4th hour, 8th hour and lead (II) acetate-5% (EDTA) at 1st hour (2000 and 4500 μM) were shown in Figure 3. Because of lead (II) acetate adsorption capacity of clinoptilolite has increased in a time dependent manner cell viability has also increased. After the treatment of clinoptilolite for 8 hours with lead (II) acetate, the sample given to the cells at concentrations of 2000 and 4500 μM maximally increased cell viability. Interestingly, even half an hour of clinoptilolite treatment is enough to increase the cell viability two-fold at both concentrations. However, cell viability at significant toxic dose (4500 μM) was not sufficient for the 8-hour lead (II) acetate-5% clinoptilolite treatment period, although the clinoptilolite increased cell viability depending on the lead (II) acetate treatment time. If lead (II) acetate treated with clinoptilolite for a longer period than 8 hours, cell viability may be further increased even toxic dose. Also, 5% (EDTA) treated lead (II) acetate at 1 hour was given to the cells. Ethylene Diamine Tetra Acetic Acid (EDTA) has significantly reduced cell viability at both concentrations (2000 and 4500 μM). Although Ethylene Diamine Tetra Acetic Acid (EDTA) adsorbs almost all of the lead (II) acetate in a short time like 1 hour, but the effect on the cells is negative. However, clinoptilolite increases cell viability even at longer adsorption time of lead (II) acetate. To confirm the cell viability test, a real-time cell analysis system was used. The xCELLigence technology is an advanced Real Time Cell Analysis (RTCA) system that allows monitoring of cell growth in real-time with the help of a label-free cell-based assay that measures the variations of impedance occurring in the culture media [23]. In this study, lead (II) acetate, (2000 and 4500 μM), lead (II) acetate 1 hour- and 8-hours treatment of 5% clinoptilolite (2000 and 4500 μM), clinoptilolite (%5) treated cells were followed for 65 hours in a real time cell analysis system. Toxic doses of the lead (II) acetate determined by the cell viability test were used to show whether clinoptilolite eliminates the toxic effect of lead or not. Toxic concentrations of lead (II) acetate (2000 and 4500 μM) treated with zeolite for 8 hours did not show cytotoxic effect on keratinocyte cells, on the contrary supported cell viability. Lead (II) acetate treated with clinoptilolite for 1 hour reduced the toxic effect of lead (II) acetate on keratinocyte cells. Longer treatment of lead (II) acetate with zeolite leads to more adsorption of lead (II) acetate, thereby reducing the toxic effect on the cells. After about 24 hours from the clinoptilolite application to the attached keratinocyte cells, the cell proliferation is exerted on the control and remained stable after 24 hours. An explanation of this finding might be that the clinoptilolite plays a major role in the decrease of the toxic effect of time-dependent clinoptilolite treated lead (II) acetate on cells. These results are directly proportional to the morphological images of the HaCaT cells. The pictures of the HaCaT cells treated with lead (II) acetate, lead (II) acetate-%5 clinoptilolite (2000 and 4500 μM), lead (II) acetate-5% Ethylene Diamine Tetra Acetic Acid (EDTA) (2000 and 4500 μM) are given in Figure 4. Cells treated with lead (II) acetate at 2000 and 4500 μM concentrations showed disturbance at the formation of the cell membrane like shrinkage, relative to untreated control. Significant difference could be observed between lead (II) acetate-5% clinoptilolite and lead (II) acetate-5% (EDTA) treated cells at 2000 and 4500 μM concentrations. While 5% clinoptilolite treated lead (II) acetate supports cell growth, Ethylene Diamine Tetra Acetic Acid (EDTA) treated decreases at the same doses. An explanation of these findings might be that clinoptilolite may have significant effects on the growth of various cells [24].
Based on these results, we conclude that while both lead (II) acetate and Ethylene Diamine Tetra Acetic Acid (EDTA) inhibit the growth of keratinocyte cells, only clinoptilolite and 5% clinoptilolite treated lead (II) acetate induces cell viability.
Further studies will be needed to determine if clinoptilolite will be suitable natural cell protecting chelator for the other heavy metals. Taking into account the central role of natural clinoptilolite in the induction of chelation potential and cell growth, it will shed light on future work.
The authors are thankful to Bekir Şahin, the chairman of the board of GLOHE for his kind contributions.
Availability of data and materials
All data generated and or analyzed during this study are included in this published article.
Citation: Sagir T, Kenar M, Ozokan G, Bilginer K (2021) In Vitro Study on Tribologically Micronized Zeolite as A Natural Cell Protecting Chelator of Lead (II) Acetate. J Clin Toxicol. 11:484.
Received: 05-Apr-2021 Accepted: 19-Apr-2021 Published: 26-Apr-2021 , DOI: 10.35248/2161-0495.21.11.484
Copyright: © 2021 Sagir T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.