Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Research Article - (2021)Volume 9, Issue 1
Oxidative stress is a critical etiologic factor and driver of inflammatory responses, witnessed in chronic and persistent conditions. The current anti-oxidative stress and anti-inflammatory drugs are associated with detrimental effects, high dependence, high costs, inaccessibility, among other drawbacks; therefore, a need for alternatives is imperative. Despite the remarkable potential of medicinal plants, there are scanty empirical studies on their pharmacologic efficacy. The Phytexponent is an alcoholic polyherbal preparation of Allium sativum, Triticum repens, Echinacea purpurea, Viola tricolor and Matricaria chamomilla. In complementary medicine, the Phytexponent is used to boost immunity, to treat inflammatory disorders, oxidative stress, blood pressure, diabetes, stress/depression, among other conditions. However, there is no sufficient scientific data to support these healing claims. Therefore, in the current study evaluated the in vitro anti-inflammatory, antioxidant activities and qualitative phytochemical composition of the Phytexponent. The in vitro anti-inflammatory activities were evaluated using the inhibition of protein denaturation and the human erythrocyte (HRBC) membrane stabilization techniques. Antioxidant activities were evaluated by the 1,1-diphenyl-picryl-1-hydrazyl (DPPH) radical scavenging-, the hydroxyl radical scavenging- and catalase activities. Qualitative phytochemical screening was performed using standard procedures. The results showed a significantly higher percentage inhibition of heat-induced- and hypotonicity induced HRBC hemolysis by the Phytexponent at concentrations of 50% and 100%, compared with the percentage inhibitions of etanercept (p<0.05). No significant differences in percentage inhibitions of protein denaturation were observed among concentrations of 12.5%,25.0%,50.0 %,100.0% of the Phytexponent and etanercept (25 mg/ml) (p
Anti-inflammatory activity; Protein denaturation; Antioxidant activity; Phytexponent; Allium sativum; Triticum repens; Echinacea purpurea; Viola tricolor and Matricaria chamomilla; Phytochemicals
Inflammation is an adaptive retort of the body to noxious stimuli like pathogens, toxins, chemicals, or physical harm [1]. Research has demonstrated a strong relationship between oxidative stress and inflammation in living systems [2,3]. Furthermore, oxidative stress has been implicated as a critical instigator and trigger of inflammatory responses [4]. Therefore, homeostatic shifts in endogenous redox status impair the proper functioning of biomolecules, immune cells and immune molecules, thereby causing undesirable events [5-7]. Oxidative stress in most cases has been linked to the genesis of most chronic and degenerative diseases that include atherosclerosis, cancer, diabetes, heart failure, liver injury, aging, chronic inflammation, neurodegenerative disorders, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and a plethora of other diseases [6-9].
Despite the phenomenal advances in conventional medicine, the current drugs prescribed for inflammation are associated with adverse side effects, are arguably inaccessible, unaffordable, and long-term use leads to tolerance and habituation [10-13]. Because of this, there is an urgent need for complementary and alternative approaches is warranted. Plants offer a viable and feasible alternative as they have for ages been an integral part of humankind in providing food, medicines, among other essential materials [3,14].
The secondary metabolites (phytochemicals), which are synthesized by medicinal plants, play critical roles in modulating the body’s redox status as well as inflammatory responses [2,15,16]. Interestingly, antioxidant phytochemicals promote health by scavenging free radicals, restoring the redox homeostasis, as well as enhancing the proper functioning of cellular biomolecules and cellular apparatus [2,3,14,17]. Additionally, the biological roles of these phytoactive plant-derived molecules in the body’s signaling pathways via their interaction with cellular enzymes, specific receptors, as well as essential transcription factors, have been demonstrated [2].
Phytexponent preparation consists of five medicinal plants, namely: Allium sativum (garlic), Triticum repens (couch grass), Echinacea purpurea (purple coneflower), Viola tricolor (wild pansy) and Matricaria chamomilla (chamomile). Allium sativum is used in traditional medicine to manage blood sugar levels, regulate blood pressure, cholesterol levels, as a stimulant of the immune system and as an anticancer medicine [17-19]. Echinacea purpurea is utilized to boost immunity, manage high blood pressure, arthritis, and cancer [20-23]. Triticum repens is active against diabetes mellitus and can lower cholesterol concentrations [24,25]. Viola tricolor has been shown to possess anticancer and anti-inflammatory properties [26,27]. The bioactive compounds of Matricaria chamomilla have been shown to exhibit antispasmodic, anxiolytic, anti-inflammatory, antimutagenic, and cholesterol-lowering effects [28-30].
Despite the application of the Phytexponent in the management of oxidative stress, inflammation among other disorders, no sufficient studies have been conducted to evaluate its pharmacologic efficiacy. The present study sought to evaluate the in vitro anti-inflammatory, antioxidant and qualitative phytochemical composition of the phytexponent as a potential therapy against oxidative stress and inflammation.
Source and Preparation of the Phytexponent
The Phytexponent preparation (Pharmapath 27, Belgium; LOT NO:17E19) was purchased from a local herbal chemist and stored according to the manufacturer’s recommendations. During experimentation, the Phytexponent was diluted in Normal saline to achieve various percentage concentrations for assay.
Determination of in vitro anti-inflammatory activity
Determination of membrane stabilization activity: The human erythrocyte membrane stabilization assay was performed as per the protacols of Sakat et al. [19] with minor modifications. Fresh blood was obtained from an individual with stable health and free from NSAIDs medication for the last two weeks prior to this study. This blood was then mixed with same mount of sterile Alseiver solutionT (2% dextrose, 0.8% sodium citrate, 0.05% citric acid, and 0.42% sodium chloride in 100 ml of water) and then centrifuged at 3000 rpm. The resultant packed human redblood cells after discarding the supernatant were rinsed with normal saline (0.9%, pH 7.0). The packed red blood cells were then reconstituted in normal saline to yield 10% v/v Human red blood cells suspension.
Hypotonicity induced hemolysis: Different concentrations of the phytexponent (3.125%, 6.25%, 12.5%, 25.0%, 50%, and 100%), reference sample, and control were separately mixed with 1ml of phosphate buffer, 2ml of hyposaline and 0.5 ml of erythrocyte suspension. Etanercept at a concentration of 25 mg/ml was used as a reference drug. All the assay mixtures were incubated at 37°C for 30 minutes and centrifuged at 3000 rpm. The supernatant liquid was aspirated, and its absorbance was measured by a Shimadzu-16001, Tokyo Japan spectrophotometer at 560 nm. The percentage of hemolysis was estimated by assuming the hemolysis produced in control is 100%. The percentage of erythrocyte membrane stabilization or protection was calculated by using the formula:
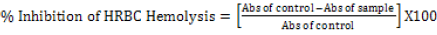 [31]
[31]
Heat-induced hemolysis: The 2ml reaction mixture comprised of 1ml phytoexponent at various study concentrations (3.125%, 6.25%, 12.5%, 25.0%, 50%, and 100%) or etanercept (reference drug at 25 mg/ml) and 1 ml of 10% v/v erthroceyte suspension. The negative control comprised of only the erythrocyte suspension and normal saline. The reaction mixtures in all tubes were incubated in athermostat set at 56°C for half an hour. Post the incubation period, all the reaction mixtures were brought to room temperature under cooled water contiously flowing and then centrifuged at 2500 rpm for 5 minutes. The resultant supernatants were specrtrophtometrically monitered at 560 nm and the absorbance recorded. All the tests for each sample was performed in triplicates. Assuming the haemolysis of the negative ccontrol as 100%, the percentage haemolysis was determined. The percentage Human red blood cells haemolysis inhibition or was tabulated a follows:
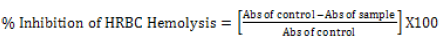 [32]
[32]
Determination of Protein Denaturation Inhibition
The anti-inflammatory activity was studied by inhibition of albumin denaturation technique, according to Mizushima & Kobayashi, [33] and Sakat et al. [33] with minor modifications. The reaction mixture was consisting of test extracts (3.125%, 6.25%, 12.5 %, 25.0%, 50%, and 100%) and 1% aqueous solution of bovine albumin fraction. The sample extracts were incubated at 37°C for 20 min and then heated to 51°C for 20 min, after cooling the samples, the turbidity was measured at 660 nm. Etanercept at a concentration of 25 mg/ml was used as a reference.The experiment was performed in triplicate. The percentage of inhibition of protein denaturation was calculated as follows:
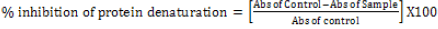 [33]
[33]
Determination of in vitro Antioxidant Activities
The DPPH radical scavenging activity: The DPPH free radical scavenging activity of the Phytexponent preparation was performed as described by Brand-Williams & Berset [34] and Ruiz-Terán et al. [35] with some modifications. The different concentrations of 100%, 10%, 1%, 0.1%, and 0.01% of the phytexponent preparation and the ascorbic acid were obtained by diluting the stock solutions in methanol. Into the differently labeled test-tubes, 2.5 ml of the different concentrations of phytexponent preparation or Ascorbic acid were added, followed by 1 ml of 0.3 mM methanolic solution of DPPH. The mixtures in all the test-tubes were then mixed by careful shaking of the test-tubes and then incubated for 15 minutes in an opaque area under room temperature. The absorbance was then recorded at 517 nm against methanol as the blank solution using a microprocessor UV-Vis double beam spectrophotometer. The control solution consisted of 2.5 mL of DPPH and 1 mL of methanol without phytexponent preparation or Ascorbic acid. The percentage of free radical scavenging activity was calculated from the equation.
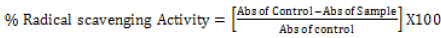 [35]
[35]
Hydroxyl radical scavenging activity: The hydroxyl radical scavenging activity of the phytexponent preparation was determined by adopting the protocol of Klein et al. [36] with minor modifications. The phytexponent preparation and reference antioxidant (L-Ascorbic acid) were diluted in normal saline to yield the different woking concentrations of 100%, 10%, 1%, 0.1%, and 0.01%. Into the different test-tubes as per the different concentrations, the reaction mixture summing up of 2.4ml of phosphate buffer (pH7.8), 90 μl of 1mM 1, 10 phenanthroline, 150μl of 0.1mM Hydrogen peroxide, 60 μl of 1mM Irion (III) chloride were added followed with 1.5ml of Phytexponent preparation and L-Ascorbic acid into the respective test-tubes. The controls contained all the reagents except the test sample (Phytexponent preparation) and the standard (L-Ascorbic acid). The reaction mixtures in all the test-tubes were carefully shaken and then incubated for five minutes under room temperature. The decrease in absorbance at 560 nm was measured, and the percentage radical scavenging activity obtained from the formula:
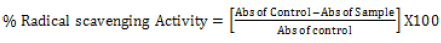 [36]
[36]
Determination of catalase enzyme activity assay: In this study, the method of Aebi [37] and modified by Atawodi [38] was followed. Briefly, 10 μl of serum was added to be added into test tubes containing 2.80 ml of 50 mM potassium phosphate buffer in different each at a time. Then, 100 μl of the Phytexponent preparation in the concentrations of 1,0.1,0.01 and 0.001% were added into the specific test tubes in triplicate. The reaction process was initiated upon the addition of 100 μl of freshly prepared 30 mM hydrogen peroxide to the reaction mixture, and the decomposition rate of hydrogen peroxide was spectrophotometrically monitored at 240 nm for 3 minutes at intervals of 1 min on a UV-VIS spectrophotometer.
Qualitative Phytochemical Screening of the Phytexponent
Qualitative phytochemical analysis for phenols, flavonoids, steroids, terpenoids, glycosides, alkaloids, tannins, saponins and anthroquinones was performed according to the methods followed by by Moriasi et al. [39,40].
Data Management and Statistical Management
In vitro, anti-inflammatory, and antioxidant activities’ data were
first tabulated on a spreadsheet using Excel (Office 365). The data was then exported to Minitab software version 19.2 for analysis.
The descriptive analysis was performed, and the values were
then presented as 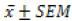 . One-Way ANOVA was performed
to determine statistical significance among groups followed by
Tukey’s test for pairwise comparisons and separation of means.
Comparisons between independent treatments were made using
the Unpaired student t-test at α.05. Means with p<0.05 were
considered statistically significant. Qualitative Phytochemical
screening data was just tabulated. The results were presented in
tables.
. One-Way ANOVA was performed
to determine statistical significance among groups followed by
Tukey’s test for pairwise comparisons and separation of means.
Comparisons between independent treatments were made using
the Unpaired student t-test at α.05. Means with p<0.05 were
considered statistically significant. Qualitative Phytochemical
screening data was just tabulated. The results were presented in
tables.
Ethical Considerations
This study was conducted as per the ethical guidelines set out by the Scientific Research Ethics Review Committee of Mount Kenya University.
In vitro Anti-Inflammatory Activity of the Phytexponent Preparation
During inflammation, digestive lysosomal enzymes are secreted to the inflamed site to counter the invader [41]. Consequently, stabilization of the lysosomal membrane helps to either delay or decrease the release of proteases among other mediators, thereby decreasing inflammation [32,42]. Since the human red blood cell (HRBC) membrane is analogous to that of the lysosome, a plant extract or compound that can inhibit hypotonicity-induced or heatinduced HRBC hemolysis can stabilize lysosomal membrane, thus averting its lysis leading to reduced inflammation [32,43].
Table 1 presents the results for the inhibition of hypotonicity and heat-induced HRBC hemolysis by the Phytexponent and the etanercept. The results showed a concentration-dependent increase in inhibition of HRBC hemolysis by the Phytexponent under hypotonicity and heat stress (Table 1).
| Treatment | Concentration | % Inhibition of Hypotonicity-induced HRBC hemolysis | % Inhibition of Heat-induced HRBC hemolysis |
|---|---|---|---|
| Phytexponent (%) | 3.125 | 32.63 ± 2.15f | 21.75 ± 1.50f |
| 6.25 | 37.07 ± 0.36f | 43.56 ± 1.30e | |
| 12.50 | 56.49 ± 0.99e | 48.68 ± 0.32d | |
| 25.00 | 65.07 ± 0.35d | 55.24 ± 0.10c | |
| 50.00 | 81.80 ± 0.13b | 71.97 ± 1.31b | |
| 100.00 | 92.23 ± 0.13a | 92.44 ± 0.41a | |
| Etanercept (Enbrel) (mg/ml) | 25 | 71.74 ± 0.33c | 50.87 ± 0.36cd |
 ; Means with different superscript alphabets within the same column are significantly different among themselves (One-Way ANOVA followed by Tukey’s test; p<0.05).
; Means with different superscript alphabets within the same column are significantly different among themselves (One-Way ANOVA followed by Tukey’s test; p<0.05).Table 1: Inhibition of hypotonicity-induced and heat-induced HRBC hemolysis by the Phytexponent.
In the hypotonicity induced HRBC hemolysis, the percentage inhibition of HRBC hemolysis recorded at 100% concentration of the Phytexponent was significantly higher compared with the percentage inhibition at lower concentrations of 3.125% and 6.25% (p˂0.05; Table 1). However, at concentrations, 3.125% and 6.25%of the Phytexponent, no significant difference in the percentage inhibition of the hemolysis of the HRBC was observed (p>0.05). Interestingly, the percentage inhibitions of hypotonicityinduced HRBC hemolysis caused by the Phytexponent at concentrations of 50% and 100% were significantly higher than the percentage inhibition caused by the reference drug (etanercept) (p˂0.05; Table 1).
Besides, a positive concentration-dependent increase in percentage inhibition of heat-induced HRBC hemolysis was depicted by the Phytexponent (Table 1). The percentage inhibition of heat-induced HRBC hemolysis by the Phytexponent at a concentration of 100 % was significantly higher than the percentage inhibitions obtained at the other concentrations (p<0.05; Table 1).
Remarkably, the percentage inhibitions of heat-induced HRBC hemolysis recorded by the Phytexponent at concentrations of 50% and 100% were significantly higher than the percentage inhibition caused by etanercept, the reference drug (p<0.05; Table 1). However, no significant difference in percentage inhibition of heat-induced hemolysis was observed between etanercept and the Phytexponent at concentrations of 12.5% and 25% (p>0.05; Table 1).
These results show a great potential of the Phytexponent in the management of inflammatory conditions in the body. Studies have shown that extracts that stabilize the hypotonicity- and heatinduced HRBC hemolysis can be good candidates for in vivo studies and subsequent drug development [5,44].
In Vitro Inhibition of Protein Denaturation by the Phytexponent
Protein denaturation involves the destruction of the tertiary and secondary structures of proteins. The denaturation is as a result of the disruption of the forces that hold these structures together when subjected to external stress or compounds, such as strong acids or bases, concentrated inorganic salts, organic solvents, or heat [45-49]. Most biological proteins lose their biological function when denatured, leading to a myriad of inflammatory conditions [49,50].
Denaturation of proteins by either the pyretic state or proteases leads to tissue injury delayed wound healing, organ dysfunction, among other associated chronic diseases like rheumatoid arthritis [51]. The production of autoantigens in certain arthritic diseases may be due to the denaturation of proteins responsible for the autoimmune responses that result in rheumatoid arthritis [42,51].
As part of the investigation on the mechanism of the antiinflammation activity, the ability of the Phytexponent to inhibit the denaturation of bovine serum albumin protein was studied with etanercept as a reference drug [31,33].
The results showed no significant differences in percentage inhibitions of protein denaturation among the Phytexponent concentrations (12.5%, 25%, 50% and 100%) and etanercept (25 mg/ml) (p>0.05; Table 2). However, at concentrations of 3.125% and 6.25%, significantly lower percentage inhibitions of protein denaturation were observed (p˂0.05; Table 2).
| Treatment | Concentration | % Inhibition of Protein denaturation |
|---|---|---|
| Phytexponent (%) | 3.125 | -340.70- ± 12.50c |
| 6.25 | 5.50 ± 1.30b | |
| 12.50 | 65.67 ± 0.67a | |
| 25.00 | 79.68 ± 0.19a | |
| 50.00 | 89.23 ± 0.23a | |
| 100.00 | 91.65 ± 0.19a | |
| Etanercept (Enbrel) (mg/ml) | 25 | 87.11 ± 0.68a |
 ; Means with similar superscript alphabet are not significantly different among themselves (One-Way ANOVA followed by Tukey’s test; p>0.05).
; Means with similar superscript alphabet are not significantly different among themselves (One-Way ANOVA followed by Tukey’s test; p>0.05).Table 2: In vitro inhibition of protein denaturation by the Phytexponent.
Research has shown that an extract or compound capable of inhibiting protein denaturation is a potent anti-inflammatory agent [49,51]. From the obtained results, the Phytexponent significantly inhibited heat-induced bovine serum albumin denaturation denoting its potential as an anti-inflammatory remedy [43-51].
Moreover, the probable mechanism through which the Phytexponent may have inhibited protein denaturation could be through the stabilization of electrostatic, hydrogen, hydrophobic, and disulfide bonding in the protein structure [32,49]. Indeed, various anti-inflammatory agents, including non-steroidal anti-inflammatory drugs, have been found to prevent protein denaturation besides preventing prostaglandin synthesis [52].
Furthermore, the tumor necrosis factor α (TNF- α) plays a central role in inflammatory processes, including the induction of collagenand cartilage-degrading metalloproteinases, which denature tissues [5,43,53]. Also, the TNF α triggers the synthesis of prostaglandins among other pro-inflammatory mediators, which all add up to the detriment of body tissues [1,54].
As a result, the inhibition of TNF α by pharmacologic agents like etanercept has been perceived as an effective therapy for inflammatory conditions [53,55]. Therefore, it is anticipated that one possible mechanism through which the Phytexponent exerted its effects could be through the inhibition of TNF α, just like the reference drug (etanercept).
In Vitro Antioxidant Activities
In vitro DPPH radical scavenging activity of the Phytexponent: Plants have, for long, been used by humans as sources of medicines to manage various ailments [56-59]. The widely acknowledged mechanism of bioactivity of medicinal plants is through the amelioration of oxidative stress [60,61]. This bioactivity is due to various antioxidant phytochemical compounds, which work is complex mechanisms to restore redox homeostasis in body cells [7,39,62-64].
The link between oxidative stress and inflammation has been explored extensively [16,65]. Antioxidant therapy has been proposed as the most viable and practical approach to preventing and reversing inflammatory processes [2,14,66]. Given this, we studied the in vitro DPPH radical scavenging activity of the Phytexponent as a measure of its antioxidant capacity.
The results showed that the percentage of radical scavenging activities produced by the Phytexponent at concentrations of 0.01%,0.1%, and 1% were not significantly different (p>0.05; Table 3). However, significantly higher percentage radical scavenging activities were observed at the Phytexponent concentration of 10% and 100 % compared with those produced at concentrations 0.01%, 0.1%, and 1 % (p<0.05; Table 3).
| Concentration (%) | % DPPH Radical Scavenging Activity | |
|---|---|---|
| Phytexponent | L-Ascorbic acid | |
| 0.01 | 68.06 ± 0.92cA | 67.71 ± 0.80bA |
| 0.1 | 70.90 ± 1.85cA | 68.06 ± 0.92bA |
| 1 | 71.34 ± 1.95cA | 70.68 ± 1.08bA |
| 10 | 80.45 ± 0.30bB | 94.09 ± 3.56aA |
| 100 | 89.69 ± 0.35aB | 99.25 ± 0.08aA |
| IC50 | 0.00733 | 0.00275 |
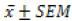 ; Means with similar lowercase superscript alphabet within the same column are not significantly different (One-Way ANOVA followed by Tukey’s test; p>0.05), while values with similar uppercase superscript alphabet across the same row are not significantly different at α0.05 (Unpaired student t-test; p>0.05)
; Means with similar lowercase superscript alphabet within the same column are not significantly different (One-Way ANOVA followed by Tukey’s test; p>0.05), while values with similar uppercase superscript alphabet across the same row are not significantly different at α0.05 (Unpaired student t-test; p>0.05)Table 3: In vitro DPPH free radical scavenging activity of the Phytexponent.
On the other hand, no significant differences among the percentage free radical scavenging activities generated by the L-Ascorbic acid at a concentration of 0.01%, 0.1%, and 1%, and between 10% and 100% were noted (p˃0.05; Table 3). However, the percentage of radical scavenging activities produced by L-ascorbic acid at concentrations 10% and 100% were significantly higher than those obtained at lower concentrations (p<0.05; Table 3).
Additionally, the concentration required to scavenge 50% of the DPPH radical (IC50) was calculated from the plot of concentration against the percentage of radical scavenging activity. The Phytexponent preparation and L-ascorbic acid had the IC50 values of 0.00733% and 0.00275%, respectively.
Research has indicated that higher the percentage of DPPH radical scavenging activity correlates to higher antioxidant activity [67- 69]. From the obtained results, the Phytexponent was considered a potent DPPH radical scavenger. Furthermore, an appraisal criterion of Blois [67] and Fidrianny et al. [70] was used to grade the antioxidant activity of the Phytexponent. Based on this criterion, the low IC50 value of the Phytexponent obtained in this study indicates remarkable antioxidant capacity [70]. The observed antioxidant effects could be due to the free radical neutralizing ability or the transfer of either a hydrogen atom or electron, which stabilize the redox equilibrium [71]
In vitro hydroxyl radical scavenging activity of the Phytexponent: Research has shown that excessive hydroxyl radicals have deleterious effects in the body ranging from increased fibrinolysis, sulfitolysis, destabilized membranes leaking to leakage, cross-linking of DNA, among others [32,42]. Furthermore, hydroxyl radicals induce major pathogenies biological membrane phospholipids in addition to their demonstrable reactivity with unsaturated fatty acids and ferrous [32,42,43]. These radicals trigger cascade reactions that alter cell integrity leading to a continuum of human pathologic conditions. These events have been implicated in neurodegenerative disorders, cancer, diabetes mellitus, arthritis, among other chronic illnesses. Hence, the current study sought to evaluate the hydroxyl radical scavenging activity of the Phytexponent [36].
The results showed a positive concentration-dependent increase in percentage hydroxyl radical scavenging activities for both the Phytexponent and L-ascorbic acid with significant differences (p<0.05; Table 4). Moreover, no significant differences between the percentage hydroxyl radical scavenging activities recorded by the Phytexponent and L-ascorbic acid at concentrations of 0.1%, 1%, and 100% (p>0.05; Table 4). However, significantly higher percentage hydroxyl radical scavenging activities were produced by L-ascorbic at concentrations of 0.01% and 10% compared with those generated by the Phytexponent at similar concentrations (p<0.05; Table 4).
| Concentration (%) | % Hydroxyl radical scavenging activity | |
|---|---|---|
| Phyt exponent | L-Ascorbic acid | |
| 0.01 | 14.60 ± 0.40eB | 28.54 ± 1.04eA |
| 0.1 | 42.00 ± 1.00dA | 44.30 ± 0.03dA |
| 1 | 53.75 ± 0.75cA | 56.15 ± 0.04cA |
| 10 | 60.75 ± 1.25bB | 70.00 ± 0.22bA |
| 100 | 73.15 ± 0.85aA | 76.00 ± 0.30aA |
| IC50 | 0.716 | 0.588 |
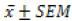 ; Means with similar lowercase superscript alphabet within the same column are not significantly different (One-Way ANOVA followed by Tukey’s test; p>0.05), while values with similar uppercase superscript alphabet across the same row are not significantly different at α0.05 (Unpaired student t-test; p>0.05)
; Means with similar lowercase superscript alphabet within the same column are not significantly different (One-Way ANOVA followed by Tukey’s test; p>0.05), while values with similar uppercase superscript alphabet across the same row are not significantly different at α0.05 (Unpaired student t-test; p>0.05)Table 4: In vitro hydroxyl radical scavenging activity of the Phytexponent.
Moreover, the concentrations of the Phytexponent and L-ascorbic acid required to scavenge 50% of the hydroxyl radicals (IC50) were determined in this study. The IC50 values were 0.588% for L-ascorbic and 0.716% for the Phytexponent (Table 4).
Based on the results, the low IC50 value depicted by the Phytexponent demonstrated the remarkable antioxidant activity as per the criterion of Fidrianny et al. [70]. Perhaps, the anti-inflammatory efficacy reported herein is due to the antioxidant activity [14].
Studies have shown that the beneficial effects of plant-derived hydroxyl scavengers could be through lipid modification by reducing the unsaturation level at double bonds [71]. Additionally, the presence of antioxidant phytocompounds works to thwart oxidative stress, thereby alleviating the underlying or associated health complications [71,72].
Effects of the Phytexponent on in vitro catalase enzyme activity: Catalase is a ubiquitous endogenous enzyme that catalyzes the disintegration of hydrogen peroxide, a toxic aerobic metabolism reactive oxygen species, which drives oxidative stress pathology [73–77]. This enzyme plays critical roles in quenching and prevention of reactive oxygen species build-up, thereby ensuring redox homeostasis. In light of this, we investigated the effects of the Phytexponent on catalase enzyme activity in vitro [37,38].
The results showed no significant difference in catalase enzyme activity at 0.001 and 0.01% of the Phytexponent (p>0.05; Table 5). Similarly, the catalase enzyme activities at concentrations of 0.1%, 0.01%, and 0.001% of L-ascorbic acid were not significantly different among themselves (p>0.05; Table 5). However, the catalase enzyme activities recorded at a concentration of 1% both the Phytexponent and L-ascorbic acid were significantly higher than catalase enzyme activities recorded at all the other concentrations (p<0.5; table 5)
| Concentration (%) | Catalase enzyme activity (u/mg protein) | |
|---|---|---|
| Phytexponent | L-ascorbic acid | |
| 1 | 62.54 ± 1.71aA | 54.82 ± 0.12aB |
| 0.1 | 56.95 ± 0.23bA | 45.57 ± 0.74bB |
| 0.01 | 44.74 ± 0.03cA | 44.72 ± 0.85bA |
| 0.001 | 44.07 ± 0.07cA | 44.12 ± 0.37bA |
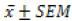 ; Means with similar lowercase superscript alphabet within the same column are not significantly different (One-Way ANOVA followed by Tukey’s test; p>0.05), while values with similar uppercase superscript alphabet across the same row are not significantly different at α0.05 (Unpaired student t-test; p>0.05).
; Means with similar lowercase superscript alphabet within the same column are not significantly different (One-Way ANOVA followed by Tukey’s test; p>0.05), while values with similar uppercase superscript alphabet across the same row are not significantly different at α0.05 (Unpaired student t-test; p>0.05).Table 5: Effects of the Phytexponent on in vitro catalase enzyme activity.
Furthermore, a comparison between the catalase activities produced at each of the studied concentrations was performed. The results showed no significant differences in catalase enzyme activities between the Phytexponent and L-ascorbic acid at concentrations of 0.001% and 0.01% (Table 5). However, the catalase enzyme activity recorded at 0.1% and 1% of the Phytexponent were significantly higher than those recorded at similar concentrations of L-ascorbic acid (p<0.05; Table 5).
These results indicate significant potentiation of catalase activity, and therefore, it is suggestive that the Phytexponent can enhance endogenous antioxidant enzymes, thus aiding remediation of oxidative stress and associated maladies [72].
Qualitative Phytochemical Composition of the Phytexponent
The medicinal value of medicinal plants is linked with the presence of secondary metabolites, the phytochemicals, synthesized by these plants [15,78,79]. In this study, the qualitative phytochemical composition of the Phytexponent was determined. The results revealed the presence of flavonoids, phenols, alkaloids, glycosides, tannins, among other pharmacologically important compounds (Table 6).
| Phytochemicals | Inference |
|---|---|
| Flavonoids | + |
| Alkaloids | + |
| Phenols | + |
| Glycosides | + |
| Steroids | + |
| Terpenoids | + |
| Tannins | + |
| Saponins | + |
| Anthraquinones | - |
| +: Present; -; Absent | |
Table 6: Qualitative phytochemical composition of the Phytexponent.
The role of medicinal plant phytochemicals in the promotion of health has been extensively deciphered [80–83]. The presence of antioxidant compounds, including flavonoids, phenols, tannins, among other phytochemicals in the Phytexponent, explains its antioxidant and anti-inflammatory efficacy reported in this study [2,72,84-86]. It is suggestive that these phytocompounds either singly or in synergy can quench oxidative stress and modulate the body’s immune response, thus modulating pathologic inflammatory evocation [2,85].
Based on the findings reported in this study, the Phytexponent has in vitro antioxidant and anti-inflammatory activities. Furthermore, the Phytexponent has anti-inflammatory and antioxidant associated phytochemicals. Therefore, the Phytexponent may be potential source of antioxidative and anti-inflammatory molecules of pharmacologic significance. This study recommends further studies aimed at isolating bioactive compounds responsible for the reported activities. Moreover, in vivo, anti-inflammatory studies should be done to determine whether the reported findings are replicable in in vivo setting. Furthermore, the specific mode(s) through which the Phytexponent exerts its bioactivity should be established.
The authors declare that there is no conflict of interest whatsoever regarding this study.
All data is included within the manuscript, and any further information is available from the author upon request.
This study did not receive any grant from any funding institution in the public or private sector.
The authors wish to acknowledge the Directorate of Research, Mount Kenya University, for availing laboratory space and equipment for this study. Mr. John Nzivo Mount Kenya University (School of Pharmacy) is acknowledged for his technical assistance.
Citation: Moriasi G, Nelson E, Twahirwa E (2020) In vitro anti-inflammatory, antioxidant and qualitative phytochemical evaluation of the Phytexponent preparation of selected plants. Adv Tech Biol Med. 8:277. doi: 10.4172/2379-1764.1000277
Received: 17-Nov-2020 Accepted: 07-Jan-2021 Published: 14-Jan-2021 , DOI: 10.35248/2379-1764.21.9.277
Copyright: © 2020 Moriasi G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.