Endocrinology & Metabolic Syndrome
Open Access
ISSN: 2161-1017
ISSN: 2161-1017
Research Article - (2025)Volume 14, Issue 1
The meta-analysis of inositol for the prevention of Gestational Diabetes Mellitus (GDM) is still a subject of debate due to issues such as small sample sizes and racial disparities. The aim of this comprehensive meta-analysis is to derive an overall effect and provide a succinct and definitive conclusion. The search was conducted up to July 2024 in international scientific databases, including PubMed, Web of Science and Embase. All meta-analyses investigating the role of inositol in preventing GDM were included in this study. Depending on the heterogeneity, both fixed and random effects models were employed to obtain pooled results. The I2 statistic and Cochrane Q test were utilized to assess the heterogeneity among studies. The quality of the included meta-analyses was evaluated using the Assessment of Multiple Systematic Reviews 2 (AMSTAR2) checklist. A total of 12 studies were included, encompassing 9,018 patients. The results indicate that inositol supplementation significantly reduced the incidence of GDM (RR: 0.37; 95% CI: 0.32, 0.42). For secondary outcomes, inositol supplementation notably decreased Fasting Plasma Glucose levels (FPG) (SMD: -1.31; 95% CI: -1.83, -0.79) and improved the one-hour Oral Glucose Tolerance Test (1 h OGTT) (SMD: -2.63; 95% CI: -3.87, -1.40) and the two-hour Oral Glucose Tolerance Test (2 h OGTT) (SMD: -0.95; 95% CI: -1.56, -0.34). The supplement also significantly reduced the risk of preterm birth (RR: 0.37; 95% CI: 0.28, 0.47) and Pregnancy-Induced Hypertension (PIH) (RR: 0.34; 95% CI: 0.25, 0.47). Notably, inositol had a significant effect on reducing the rate of cesarean section (RR: 0.82; 95% CI: 0.71, 0.94). However, the impact on the incidence of macrosomia was not statistically significant (RR: 0.70; 95% CI: 0.33, 1.49). The meta-analysis also found that birth weight (SMD: -0.25; 95% CI: -0.32, -0.17) and the incidence of neonatal hypoglycemia were significantly reduced (RR: 0.30; 95% CI: 0.08, 1.21). Inositol supplementation had no significant effect on gestational age at birth (SMD: -0.13; 95% CI: -0.04, 0.29). The findings of this study support the effectiveness of inositol supplementation in the prevention of GDM.
Inositol; Gestational Diabetes Mellitus (GDM); Meta-analysis of meta-analyses; Periconceptional nutritional intervention; Fetal and maternal health outcomes
Gestational Diabetes Mellitus (GDM) is characterized as glucose intolerance that is first recognized during pregnancy, with blood glucose levels that are elevated above normal but do not meet the criteria for overt diabetes. The incidence of GDM is increasing, impacting approximately 16.5% of all pregnancies globally and is associated with significant health complications. GDM confers an augmented risk of maternal complications, including gestational hypertension, preeclampsia and obstetric interventions such as cesarean section. Furthermore, offspring born to mothers with GDM are at an elevated risk for a range of adverse outcomes, including obesity, metabolic syndrome, type 2 diabetes and cardiovascular diseases later in life. Although interventions such as lifestyle changes, insulin therapy and oral hypoglycemic agents like metformin have demonstrated efficacy in managing GDM, adherence to these treatments can be challenging and there are lingering concerns about the potential long-term effects of oral medications on the fetus. Consequently, there is a burgeoning interest in the role of nutritional supplements as a primary preventive modality for GDM [1].
Inositol, a group of nine stereoisomers, is pivotal in cellular signaling pathways, with myo-inositol and D-chiro-inositol being the most biologically relevant. These isomers are interconvertible through the action of specific epimerase enzymes and are implicated as second messengers in insulin signaling cascades, modulating insulin sensitivity and glucose homeostasis through the formation of inositol polyphosphates. Empirical evidence suggests that inositol supplementation may attenuate insulin resistance, thereby reducing the risk of GDMrelated complications such as preeclampsia, preterm birth, macrosomia and neonatal hypoglycemia. However, the efficacy of inositol supplementation in the prevention of GDM remains a contentious issue, with some Randomized Controlled Trials (RCTs) reporting a significant reduction in GDM incidence in the inositol-supplemented groups compared to controls, while others, such as the study by Fraticelli F et al., have not observed such a protective effect. The heterogeneity in study outcomes underscores the need for further research involving diverse phenotypic and ethnic cohorts to elucidate the role of inositol in GDM prevention.
The objective of the current meta-analysis of meta-analyses is to critically assess the efficacy of inositol supplementation in the context of GDM prevention, aiming to provide a more nuanced understanding of its potential benefits and to address the existing discrepancies in the literature.
This study adheres to the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. The protocol for this systematic review and metaanalysis has been prospectively registered on the PROSPERO database. (Registration number: CRD42024572199).
Search strategy
A comprehensive literature search was conducted across PubMed, Web of Science and Embase databases to identify meta-analyses pertinent to inositol therapy for Gestational Diabetes Mellitus (GDM). The search was executed up to the 21st of July, 2024, inclusive and was supplemented by scrutinizing the bibliographies of eligible studies for additional relevant publications. The search strategy incorporated a blend of MeSH terms and keywords, with the complete methodology delineated [2].
Eligibility criteria
Inclusion criteria were as follows:
Exclusion criteria were applied to studies that did not conform to the "meta-analysis" design.
Methodological quality assessment and evidence grading
The methodological rigor and quality of the included metaanalyses were independently assessed by two reviewers (RTW and MYW) using the Appraisal of Multiple Systematic Reviews (AMSTAR 2) tool. The AMSTAR 2 tool comprises 16 items, which are scored as "yes," "partially," "no," or "not applicable." the quality of evidence was categorized into four levels: "very low," "low," "moderate," and "high" (Table 1).
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Overall |
| Chan KY, 2021 | √ | √ | √ | √ | √ | √ | √ | √ | × | √ | √ | √ | × | × | × | × | Moderate |
| Chaolin Li, 2024 | √ | × | √ | √ | √ | √ | √ | × | × | √ | √ | √ | √ | √ | × | × | High |
| Dorina Greff, 2023 | √ | × | √ | × | √ | √ | √ | √ | × | √ | √ | √ | √ | × | × | × | High |
| Guo X, 2018 | √ | × | √ | × | √ | × | × | × | × | √ | × | × | × | × | × | × | Low |
| Liang Li, 2022 | √ | × | √ | × | × | × | × | × | × | √ | × | × | × | × | × | × | Low |
| Patricia Ann factor, 2023 | √ | × | √ | √ | √ | √ | √ | × | √ | √ | √ | √ | √ | × | √ | × | High |
| Qinxin Liu, 2022 | √ | × | × | × | √ | √ | × | √ | × | √ | √ | √ | √ | × | × | × | Moderate |
| Sepideh Mashayekh Amiri, 2022 | √ | × | √ | × | √ | √ | × | √ | √ | √ | √ | √ | √ | √ | × | × | High |
| Vitagliano A, 2019 | √ | × | × | × | √ | √ | × | √ | √ | √ | √ | × | × | × | × | × | Moderate |
| Wei J, 2022 | √ | × | √ | × | × | × | × | √ | × | √ | √ | √ | × | √ | × | × | Low |
| Zhang, 2018 | √ | × | × | × | × | × | × | √ | × | √ | √ | × | × | × | × | × | Low |
| Zheng Xiangqin, 2015 | × | × | √ | × | √ | √ | √ | √ | × | √ | √ | × | × | √ | × | × | Moderate |
| Note: 1. Did the research questions and inclusion criteria for the review include the components of PICO? 2. Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review, and did the report justify any significant deviations from the protocol? 3. Did the review authors explain their selection of the study designs for inclusion in the review? 4. Did the review authors use a comprehensive literature search strategy? 5. Did the review authors perform study selection in duplicate? 6. Did the review authors perform data extraction in duplicate? 7. Did the review authors provide a list of excluded studies and justify the exclusions? 8. Did the review authors describe the included studies in adequate detail? 9. Did the review authors use a satisfactory technique for assessing the Risk of Bias (RoB) in individual studies that were included in the review? 10. Did the review authors report on the sources of funding for the studies included in the review? 11. If meta-analysis was performed, did the review authors use appropriate methods for the statistical combination of results? 12. If a meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? 13. Did the review authors account for RoB in individual studies when interpreting/ discussing the review results? 14. Did the review authors provide a satisfactory explanation for and discussion of any heterogeneity observed in the review results? 15. If they performed quantitative synthesis, did the review authors conduct an adequate investigation of publication bias (small-study bias) and discuss its likely impact on the review results? 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? |
|||||||||||||||||
Table 1: The results of quality assessment included meta-analyses based on AMSTAR2 questionnaire.
Study selection and data extraction
The initial screening of titles and abstracts was performed by two reviewers (RTW and LJH) based on the predefined eligibility criteria. Full texts of potentially eligible studies were retrieved and independently evaluated by the same reviewers for final inclusion. Discrepancies were resolved through consensus or by consultation with a third reviewer (XCL). Data extracted from the included studies included publication year, sample size, geographical location of the study, duration of inositol supplementation, Standardized Mean Differences (SMD), Odds Ratios (OR), Relative Risks (RR) and their corresponding 95% Confidence Intervals (CIs). ORs were converted to RRs for consistency in data analysis [3].
Statistical analysis
Statistical analyses were conducted using STATA version 16.0 (STATA Corporation, College Station, TX, US). A randomeffects model was employed to account for heterogeneity among studies when I2 exceeded 50%. This model assumes variability in effect sizes due to differences in study populations, interventions and outcomes. The heterogeneity was quantified using the I2 statistic and sensitivity analyses were performed to assess the robustness and stability of the meta-analytic findings, with a 95% Confidence Interval (CI) used for all estimates.
Study selection
Figure 1 delineates the systematic review's methodological workflow. The preliminary database search retrieved a cumulative total of 91 studies from PubMed (n=26), Web of Science (n=36) and Embase (n=29), with 41 studies identified as duplicates. After a rigorous screening process of the titles and abstracts of the remaining 50 articles, a full-text evaluation was conducted on 21 articles, yielding 19 articles that fulfilled the criteria for qualitative synthesis. For the quantitative synthesis, a total of 12 studies were ultimately included, denoted by their respective authors and publication years [4].
Figure 1: The process study selection shown on PRISMA flow chart.
Study characteristics
The curated selection of 12 studies comprised a participant pool of 9,018 women. The studies meeting the inclusion criteria spanned the period from 2015 to 2024. These investigations were geographically distributed across four countries: China (8 studies), Hungary (1 study), The Philippines (1 study) and Iran (1 study). Each of the included randomized controlled trials employed inositol as an adjunctive therapy to standard and conventional pharmacological treatments for gestational diabetes. Table 2 delineates the salient features of the studies incorporated into the meta-analysis.
| Study, Year, Country | Participants and case | Study number | Therapy | Outcome | Quality |
| Chan KY, 2021, China | Myo-inositol and D-chiro-inositol 681 |
2 | 1. Myo-inositol 2. D-chiro-inositol |
GDM rate FPG 1 h OGTT 2 h OGTT |
Yes ( Allocation; Random; Incomplete; Selective) No (Blinding) Moderate |
| Chaolin Li, 2024, China |
Myo-inositol 1319 |
4 | 1. 4 g MI+400 mg folic acid 2. 1.1 g MI+27.6 mg DCI+400 µg folic acid 3. 2 g MI 4. 4 g MI+400 µg folic acid |
GDM rate FPG 1 h OGTT 2 h OGTT PIH Preterm birth Infants with neonatal hypoglycemia |
Yes ( Allocation; Random; Incomplete) No ( Blinding; Selective) Low |
| Dorina Greff, 2023, Hungary | Myo-inositol 1357 |
5 | 1. 4 g MI+400 mcg folic acid 2. 1100 mg MI+27.6 mg DCI 3. 4 g MI+40 mcg folic acid 4. 2 g MI+200 mcg folic acid 5. 1100 mg MI+27.6 mg DCI+400 mcg folic acid |
GDM rate FPG 1 h OGTT 2 h OGTT Preterm birth pregnancy-induced Hypertensive Neonatal hypoglycemia |
Yes ( Allocation; Random; Incomplete; Blinding; Selective) High |
| Guo X, 2018, China | Myo-inositol 686 |
1 | 1. 2 g myo-inositol+200 mg folic acid | GDM rate | Yes (Allocation; Random; Incomplete; Selective) No (Blinding) |
| Liang Li, 2022, China | Myo-inositol D-Chiro inositol 1250 |
3 | 1. 2 g myo-inositol+200 µg folic acid 2. 4 g myo-inositol+400 µg offolic acid 3. 1100 mg myo-inositol, 27.6 g D-Chiro inositol and 400 mcg folic acid |
GDM 2 h OGTT Preterm delivery |
Yes ( Allocation; Random; Incomplete; Selective; Blinding) High |
| Patricia Ann Factor, 2023, Philippine |
Myo-inositol D-Chiro inositol 586 |
3 | 1. 4 g myo-inositol+400 mg folic acid 2. 2 g myo-inositol+200 mcg folic acid 3. 1100 mg myo-inositol+27.6 mg D-chiro inositol+ 400 mcg folic acid |
GDM rate Cesarean Section Pregnancy-induced hypertension pre-term birth |
Yes ( Allocation; Random) No (Blinding; Incomplete; Selective) Low |
| Qinxin Liu, 2022, China | Myo-inositol 344 |
4 | 1. 2 g myo- inositol+200 mg folic acid 2. 4 g myo-inositol+400 µg folic acid 3. 1100 mg myo-inositol, 27.6 g D. Chiro inositol 4. 2 g myo-inositol+200 µg folic acid |
GDM rate 2 h OGTT Preterm delivery Gestational age at birth birth weight |
Yes (Allocation; Random; Incomplete; Selective) No (Blinding) Moderate |
| Sepideh Mashayekh Amiri, 2022, Iran |
Myo-inositol 720 |
1 | 1. 2 g MI+200 mcg folic | GDM rate FPG 1 h OGTT 2 h OGTT Gestational hypertension Caesarian section Preterm delivery Macrosomia Neonatal hypoglycemia |
Yes (Allocation; Random; Selective) No (Blinding; Incomplete) Low |
| Vitagliano A, 2019, Italy | Myo-inositol D-Chiro inositol 448 |
3 | 1. 2 g MYO+200 μg folic acid 2. 2 g MYO+400 μg folic acid+400 mg DCI 3. 1100 mg MYO+27.6 g DCI+400 μg folic acid |
GDM rate FPG 1 h OGTT 2 h OGTT Hypertensive disorders Preterm delivery |
Yes (Allocation; Random; Incomplete; Selective) No (Blinding) Moderate |
| Wei J, 2022, China | Myo-inositol D-Chiro inositol 671 |
4 | 1. 2 g MI+ 200 µg folic acid 2. 1.1 g MI, 27.6 mg DCI+400 µg folic acid 3. 500 mg DCI +400 µg folic acid 4. 0.55 g MI, 13.8 mg DCI+ 200 µg folic acid |
GDM rate FPG 1 h OGTT 2 h OGTT |
Yes (Allocation; Random; Incomplete) No (Blinding; Selective) Low |
| Zhang, 2018, China | Myo-inositol D-Chiro inositol 432 |
2 | 1. 1100 mg myo-inositol, 27.6 g D-Chiro inositol and 400 mcg folic acid 2. 2 g myo-inositol+200 mcg folic acid |
GDM rate Preterm delivery 2 h OGTT Gestational age at birth birth weight Macrosomia |
Yes (Allocation; Random; Selective) No (Blinding; Incomplete) Low |
| Zheng Xiangqin, 2015, China | Myo-inositol 524 |
2 | 1. 2 g myo-inositol+200 mg folic acid 2. 4 g myo-inositol+400 mg folic acid |
GDM rate Birth weight FPG 1 h OTGG 2 h OTGG |
Yes (Allocation; Random; Incomplete; Selective) No (Blinding) Moderate |
Table 2: Characteristics of the meta-analysis investigating the impact of inositol supplementation on gestational diabetes.
Meta-analysis of meta-analysis results
Incidence of Gestational Diabetes Mellitus (GDM): The metaanalysis encompassed 12 studies that reported on the incidence of GDM. A fixed-effects model was applied for the meta-analysis. The results revealed a significant effect of inositol supplementation on reducing the incidence of GDM (RR: 0.37; 95% CI: 0.32, 0.42). No significant heterogeneity was observed among the studies (I2=0%, p-heterogeneity=0.764; Figure 2). The funnel plot did not indicate the presence of publication bias (Figure 3).
 Figure 2: GDM rate.
Figure 2: GDM rate. 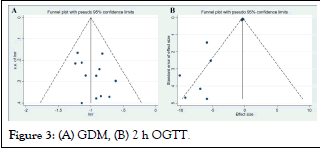 Figure 3: (A) GDM, (B) 2 h OGTT.
Figure 3: (A) GDM, (B) 2 h OGTT.
Fasting Plasma Glucose (FPG): Seven studies were identified that reported on Fasting Plasma Glucose (FPG) levels. Significant heterogeneity was detected among these studies (I2=90.8%, pheterogeneity< 0.001), necessitating the use of a random-effects model for the meta-analysis. The pooled analysis demonstrated a significant effect of inositol supplementation on FPG levels in patients with GDM (SMD: -1.31, 95% CI: -1.83, -0.79; Figure 4A). Sensitivity analysis was conducted to explore the source of heterogeneity, revealing that the study by Dorina Greff et al., deviated significantly from the other studies (Figure 5B). Upon exclusion of this study, the sensitivity analysis maintained the significant effect of inositol supplementation on FPG levels (SMD: -1.31, 95% CI: -1.83, -0.79; Figure 6C), with significant heterogeneity still observed (I2=92%, p-heterogeneity<0.001) [5].
One-hour Oral Glucose Tolerance Test (1 h OGTT): Seven studies were identified that reported on the One-Hour Oral Glucose Tolerance Test (1 h OGTT). Significant heterogeneity was noted among these studies (I2=87.3%, p-heterogeneity<0.001), prompting the use of a random-effects model for the meta-analysis. The results indicated a significant effect of inositol supplementation on reducing the 1 h OGTT glucose levels in patients with Gestational Diabetes Mellitus (GDM) (SMD: -2.63; 95% CI: -3.87, -1.40; Figure 4B). A sensitivity analysis was conducted to identify potential sources of heterogeneity, revealing that the study by Chaolin Li et al., exhibited a significant deviation from the other studies (Figure 5A). Upon exclusion of this study, the sensitivity analysis continued to show a significant effect of inositol supplementation on 1h OGTT glucose levels (SMD: -1.75, 95%CI: -2.81, -0.69; Figure 6A), with significant heterogeneity still present (I²=83.8%, p-heterogeneity<0.001).
Preterm birth: Eight studies were identified that reported on the incidence of preterm birth. No heterogeneity was detected among these studies (I2=0.0%, p-heterogeneity=0.999), allowing for the use of a fixed-effects model for the meta-analysis. The results demonstrated a significant effect of inositol supplementation on reducing the risk of preterm birth in patients with Gestational Diabetes Mellitus (GDM) (RR: 0.37; 95% CI: 0.28, 0.47; Figure 4C).
Macrosomia: Two studies were identified that reported on the incidence of macrosomia. No heterogeneity was detected among these studies (I2=0.0%, p-heterogeneity=0.868), which justified the use of a fixed-effects model for the meta-analysis. The results indicated that the effect of inositol supplementation on reducing the incidence of macrosomia in patients with Gestational Diabetes Mellitus (GDM) was not statistically significant (RR: 0.70; 95% CI: 0.33, 1.49; Figure 4D).
Preeclampsia (PIH): Five studies were identified that reported on Preeclampsia (PIH). No heterogeneity was detected among these studies (I2=0.0%, p-heterogeneity=0.925), which justified the use of a fixed-effects model for the meta-analysis. The results indicated a significant effect of inositol supplementation on reducing the risk of PIH in patients with Gestational Diabetes Mellitus (GDM) (RR: 0.34; 95% CI: 0.25, 0.47; Figure 4E).
Birth weight: Two studies were identified that reported on birth weight. Assessment of heterogeneity indicated no significant variability among the studies (I2=11.5%, p-heterogeneity=0.323), which supported the use of a fixed-effects model for the metaanalysis. The results demonstrated a significant effect of inositol supplementation on reducing birth weight in infants born to women with Gestational Diabetes Mellitus (GDM) (SMD: -0.25; 95% CI: -0.32, -0.17; Figure 4F) [6].
Two-hour Oral Glucose Tolerance Test (2 h OGTT): Ten studies were identified that reported on the Two-Hour Oral Glucose Tolerance Test (2 h OGTT). Significant heterogeneity was detected among these studies (I²=80.7%, pheterogeneity< 0.001), which necessitated the use of a randomeffects model for the meta-analysis. The results indicated a significant effect of inositol supplementation on reducing the 2 h OGTT glucose levels in patients with Gestational Diabetes Mellitus (GDM) (SMD: -0.95; 95% CI: -1.56, -0.34; Figure 4G).
A sensitivity analysis was conducted to explore the source of heterogeneity, revealing that the study by Sepideh Mashayekh Amiri et al., exhibited a significant deviation from the other studies (Figure 5C). Upon exclusion of this study, the sensitivity analysis continued to show a significant effect of inositol supplementation on 2 h OGTT glucose levels (SMD: -0.77, 95% CI: -1.32, -0.22; Figure 6B), with significant heterogeneity still present (I2=78.4%, p-heterogeneity<0.001).
Infants with neonatal hypoglycemia: Three studies were identified that reported on the incidence of neonatal hypoglycemia in infants. Assessment of heterogeneity indicated no significant variability among the studies (I2=11.5%, pheterogeneity= 0.323), supporting the use of a fixed-effects model for the meta-analysis. The results indicated a significant effect of inositol supplementation on reducing the incidence of neonatal hypoglycemia in infants born to mothers with Gestational Diabetes Mellitus (GDM) (RR: 0.30; 95% CI: 0.08, 1.21; Figure 4H).
Gestational age at birth: Two studies were identified that reported on the gestational age at birth. Assessment of heterogeneity indicated no significant variability among the studies (I2=0.0%, p-heterogeneity=0.502), which justified the use of a fixed-effects model for the meta-analysis. The results indicated that the effect of inositol supplementation on gestational age at birth in infants of mothers with Gestational Diabetes Mellitus (GDM) was not statistically significant (SMD: -0.13; 95% CI: -0.04, 0.29; Figure 4I).
Caesarian section: Two studies were identified that reported on the rate of caesarian section. Assessment of heterogeneity indicated no significant variability among the studies (I2=0.0%, p-heterogeneity=0.330), which justified the use of a fixed-effects model for the meta-analysis. The results demonstrated a significant effect of inositol supplementation on reducing the rate of caesarian section in patients with Gestational Diabetes Mellitus (GDM) (RR: 0.82; 95% CI: 0.71, 0.94; Figure 4J).
Figure 4: (A) FPG, (B) 1 h OGTT, (C) Preterm birth, (D) Macrosomia, (E) PIH, (F) Birth weight, (G) 2 h OGTT, (H) Infants with neonatal hypoglyce, (I) Gestational age at birth, (J) Caesarian section.
Figure 5: (A) 1 h OGTT, (B) FPG, (C) 2 h OGTT.
Figure 6: (A)1 h OGTT, (B)2 h OGTT, (C)FGP.
To date, the management of obesity and insulin resistance during pregnancy has relied solely on diet and lifestyle modifications, with insulin remaining the only therapeutic option for controlling Gestational Diabetes Mellitus (GDM). It is crucial to identify novel and effective strategies for the prevention of GDM to circumvent the side effects associated with current treatments. One promising example of such a strategy may be the supplementation with inositol. Previous studies have suggested encouraging effects of inositol supplementation in the prevention of GDM, indicating that this therapeutic approach may be a promising strategy. In this context, several meta-analyses of observational and Randomized Controlled Trials (RCTs) have been conducted to assess whether inositol supplementation can prevent GDM. However, existing meta-analyses and related RCTs are still limited by small sample sizes and a lack of ethnic diversity. The prevention of GDM by inositol remains a contentious issue. This study is an umbrella review of 12 meta-analyses assessing the efficacy of inositol in preventing GDM and related conditions [7].
In the present study, based on the results of the meta-analyses, we found that inositol supplementation can significantly alleviate GDM and related symptoms. However, there is heterogeneity among different meta-analyses. The high heterogeneity observed should be cautiously interpreted, especially after conducting sensitivity analyses, as significant heterogeneity still persists. Despite the significant overall effect observed in previous meta-analyses, some controversies remain. The discrepancies in the results can be attributed to different treatment dosages and durations, different types of analyses, varying meta-analysis quality and different sample sizes.
In the included meta-analyses, we assessed the quality of the studies using the AMSTAR 2 checklist. This checklist comprises 16 items that cover various aspects of meta-analysis. Among all included studies, there were 4 high-quality meta-analyses, 4 moderate-quality meta-analyses and 4 low-quality meta-analyses, indicating that our meta-umbrella results should be interpreted with caution; thus, more research is needed to draw conclusive results.
GDM is a multifactorial disease with both genetic and environmental factors playing crucial roles in its pathogenesis, leading to maternal and fetal circulatory disorders and neonatal complications. Current guidelines recommend treatments with insulin, metformin and the like. The mechanism of these drugs is to maintain blood glucose concentrations within the normal range. However, the potential side effects of these drugs on the fetus remain unknown. Therefore, preventing the occurrence of GDM is of paramount importance. A novel strategy for preventing GDM is the intake of nutrients such as inositol [8].
Inositol is a key molecule in the insulin signaling pathway, Activating Protein Kinase B (AKT) by promoting the conversion of phosphatidylinositol, thereby regulating glucose uptake and glycogen synthesis. Different forms of inositol, especially Myo- Inositol (MI) and D-Chiro-Inositol (DCI), play distinct roles in glucose metabolism, with MI primarily promoting glucose uptake and DCI being involved in glycogen storage. The use of insulin sensitizers such as inositol may help improve insulin resistance and the associated hyperandrogenic phenotype. An increasing body of evidence suggests that inositol plays a role in the treatment of various conditions, including Polycystic Ovary Syndrome (PCOS), Gestational Diabetes (GDM) and in the prevention of neural tube defects. In recent years, with growing attention to the prevention of DGM, some meta-analyses have indicated that the effect of inositol in preventing GDM is similar to that of metformin. Concurrently, inositol alone or in combination with probiotics and a Mediterranean diet has achieved remarkable results in preventing GDM [9].
Our study has several strengths. To our knowledge, the current study is the first comprehensive analysis of the impact of inositol supplementation on GDM. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, enhancing the transparency and reproducibility of our research. However, there are also some limitations in this study. The number of included studies is limited and there is a certain degree of publication bias. We recommend further meta-analytic research in this area. The quality of primary studies included in the meta-analysis is mixed and the quality of the meta-analysis is moderate; thus, highquality studies are needed in the future. Additionally, we suggest further meta-analyses to separately evaluate myo-inositol or Dchiro- inositol, as these two supplements may have different effects. We also recommend further meta-analyses to assess different treatment dosages. Since the main population included in the meta-analysis is GDM patients, we cannot assess the impact of inositol on other reproductive diseases in women; therefore, we recommend future research on this to better understand the effects of inositol.
This study systematically reviewed and synthesized 12 metaanalyses from the current literature to assess the efficacy of inositol supplementation as an intervention for the treatment of Gestational Diabetes Mellitus (GDM). Our findings indicate that inositol supplementation is associated with a significant reduction in the incidence of GDM and also shows a positive impact on improving secondary outcome measures in GDM patients, such as Fasting Plasma Glucose (FPG), one-hour and two-hour Oral Glucose Tolerance Tests (1 h OGTT and 2 h OGTT), preterm birth, Pregnancy-Induced Hypertension (PIH), neonatal hypoglycemia and the rate of caesarian section.
However, the conclusions of this study should be interpreted in the context of the methodological quality and heterogeneity of the research. Based on the assessment using the AMSTAR 2 tool, the quality of the included meta-analyses varied, indicating that our interpretation of the results should be cautious and that more high-quality studies may be needed to confirm the findings. Moreover, the significant heterogeneity observed in this study may stem from various factors, including different inositol dosages, treatment durations, study designs and differences in participant baseline characteristics.
Despite the aforementioned limitations, our study results provide robust evidence for the potential of inositol as a preventive and therapeutic agent for GDM. As a safe and potentially effective nutritional supplement, the underlying mechanisms of inositol in improving insulin sensitivity and reducing blood glucose levels offer new perspectives for the management of GDM. Furthermore, considering the long-term impacts of GDM on maternal and fetal health, inositol supplementation, as a non-pharmacological intervention, may provide a novel strategy for the prevention of GDM and its complications.
Future research should focus on well-designed, adequately powered, multicenter, randomized controlled clinical trials to evaluate the effects of different dosages and types of inositol supplementation on the prevention and treatment of GDM. Additionally, research should address the long-term safety of inositol supplementation, cost-effectiveness analysis and its generalizability across different ethnicities and geographical regions. Through these studies, we can gain a more comprehensive understanding of the role of inositol in GDM management and provide more precise guidance for clinical practice.
In summary, this umbrella review of meta-analyses provides scientific evidence for the potential application of inositol supplementation in the prevention and treatment of GDM, but it also emphasizes the need for more high-quality research to further validate these findings and to provide a more solid foundation for the implementation of inositol supplementation in GDM management.
Rutong Wang: Data curation; investigation; resources; data organization; writing-original draft (lead author); conceptualization; formal analysis.
Yanhong Wei: Data organization; investigation; writing-original draft.
Bingsheng Huang: Investigation; writing-original draft.
Weihua Nong and Xiaocan Lei: Conceptualization; methodology; project administration; supervision; writing-review and editing.
Not applicable.
No animals/humans were used in this study.
The data and supportive information are available in the article.
This work was supported by the 2022 scientific research and technology development program of Baise city (no. 20224124) and hunan province innovation and entrepreneurship training program for college students (no. s202410555233).
The authors declare no financial or other conflicts of interest.
Thanks are extended to Clinical Anatomy and Reproductive Medicine Application Institute, Hengyang Medical School, University of South China, for their assistance.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Wang R, Wei Y, Wang M, Huang L, Guo Y, Weihua N, Lei X (2025) Efficacy of Inositol Supplementation in Preventing Gestational Diabetes Mellitus: A Meta-Analysis of Meta-Analyses. Endocrinol Metab Syndr. 14:439.
Received: 09-Aug-2024, Manuscript No. EMS-24-33455; Editor assigned: 14-Aug-2024, Pre QC No. EMS-24-33455 (PQ); Reviewed: 28-Apr-2024, QC No. EMS-24-33455; Revised: 03-Jan-2025, Manuscript No. EMS-24-33455 (R); Published: 10-Jan-2025 , DOI: 10.35248/2161-1017.25.14.439
Copyright: © 2025 Wang R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.