
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2014) Volume 3, Issue 1
#
<Keywords: Gongronema latifolium; Antioxidant; n-butanol; CCl4; Histology
Gongronema latifolium (Asclepiadaceae), is a perennial climber forest leafy vegetable with woody hollow glaborous stems below and characterized by greenish yellow flowers [1]. It is widespread in tropical Africa such as Senegal, Chad and DR Congo as well as grows in the forest of south eastern and western Nigeria where it is widely used for medicinal and nutritional purposes [2]. G. latifolium occurs in rainforest, deciduous and secondary forests, and also in mangrove and disturbed roadside forest, from sea-level up to 900 m altitude. In Nigeria, information available from the indigenous traditional healers claimed that a decoction of the chopped [3] leaves of G. latifolium has been used in the production of several herbal products which are taken orally [1] for the treatment of stomach upsets and pains, dysentery, malaria, typhoid fever, worm and cough [4]. Asthma patients chew fresh leaves to relieve wheezing [1] and a decoction of the roots, combined with other plant species, is taken to treat sickle cell anaemia. A maceration of the leaves in alcohol is taken to treat bilharzia, viral hepatitis and as a general antimicrobial agent [5]. It is also taken as a tonic to treat loss of appetite [4]. Previous studies have revealed that other plants with polyphenols exhibit clear anti-hepatotoxic properties [1], and that flavonoids could protect the liver against oxidative injury induced by CCl4 in vivo [4]. Although many other plants have been reported to possess anti-hepatotoxic properties, the scientific authentication of most of them such as G. latifolium which is used traditionally to treat several diseases is unavailable [3]. The qualitative phytochemicals screening of the methanolic leave extract of G. latifolium revealed the presence of glycosides, alkaloids, saponin, flavonoids, tannins, and the absence of free anthraquinone. The quantitative analysis of phytochemical constituents of G. latifolium leaves is presented in Table 1. The crude extract showed high tannin content followed by glycosides, alkaloids and saponin. The results in Table 2 also showed that the n-butanol fraction has higher flavonoids, polyphenols and ascorbic acid content than the ethylacetate fraction. The aim of this work is to provide some scientific support for the health benefit of G. latifolium. To achieve this, studies were carried out to investigate the phytochemical constituents of G. latifolium and to evaluate the antihepatotoxic activities of n-butanol fraction of methanolic leave extract of G. latifolium against oxidative damage induced by CCl4 in Wistar albino rats.
| Leave (mg/g) | Alkaloids (mg/g) | Saponins | Glycosides (mg/g) | Tannins (mg/g) |
|---|---|---|---|---|
| Crude | 1.26 | 0.82 | 2.57 | 10.60 |
Table 1: Quantitative Analysis of the Phytochemical Constituents (mg/g) of G. latifolium
| Fractions | Polyphenols (mg/g) | Flavonoids (mg/g) | Ascorbic acid (mg/g) |
|---|---|---|---|
| n-Butanol | 4.53 | 5.15 | 2.24 |
| Ethylacetate | 2.39 | 4.51 | 0.62 |
Table 2: Quantitative Analysis of the Phytochemical Constituents (mg/g) of fractions of G. latifolium
Chemicals/reagents
All assays kits were from Randox Laboratories Ltd. Ardmore, Co. Antrm UK. Chemicals and reagents used were purchased from Sigma Chemical Company St. Louis U.S.A. and chemicals used were of analytical grade. Folin ciocalteu phenol reagent, gallic acid, carbon tetrachloride (Sigma-Aldrich), distilled water, normal saline.
Plant material and extraction
Fresh leaves (blend) of G. latifolium were obtained from a homestead garden at Isuofia, Aguata L.G.A., Anambra State, Nigeria in the month of February 2013 and authenticated at the herbarium unit by Gallah U.J. in the Department of Biological Sciences, Ahmadu Bello University, Zaria, Kaduna State, Nigeria where a voucher specimen with voucher number 1274 was deposited. The collected plants were rinsed in clean water and air dried at room temperature for two weeks. The dried leaves were pulverized into powder using Thomas- Wiley laboratory mill (model 4) manufactured by Arthur H. Thomas Company, Philadelphia, PA., U.S.A. before being extracted. A portion of five hundred grams (500 g) of the pulverized plant leaves was suspended in 2.5 L of methanol for 48 hours in large amber bottles with intermittent shaking. At the end of the extraction, the crude methanol extract was filtered using Whatmann No. 1 filter paper (1mm mesh size) and then concentrated in a water bath maintained at 45°C until greenish black residues were obtained. Certain gram of the crude extract was then subjected to phytochemical analysis using standard procedures [6]. Also, 51 g of the crude extract was reconstituted with 250 ml of methanol for further fractionation and the fractions were kept in sealed containers and refrigerated at 2-4ºC for further use. The percentage yield of both the crude methanol leaves extract and fractions were determined as a percentage of the weight (g) of the extract to the original weight (g) of the dried sample used.
Fractionation of crude extract
The crude extract of G. latifolium was subjected to liquid- liquid partition separation to separate the extract into different fractions. 250 ml of the reconstituted extract was placed in a separator funnel and 250 ml of n-hexane, ethylacetate and n-butanol solvents were added sequentially as a 1:1 (v/v) solution and rocked vigoriously [7]. The sample was left standing for 30 minutes for each solvent on the separator funnel until a fine separation line appear clearly indicating the supernatant from the sediment before it was eluted sequentially. The process was repeated thrice in order to get adequate quantity for each fraction. The n-hexane, ethylacetate, n-butanol as well as the aqueous residue fractions were evaporated to dryness in a water bath to afford four fractions in (grams) respectively.
Preliminary phytochemical screening
Test for Glycosides was carried out according to the method of Trease and Evans, 1983 [8].
Test for Anthraquinones derivatives was carried out according to the method of Trease and Evans, 1983 [8].
Test for Saponins was carried out according to the method of Trease and Evans, 1983 [8].
Test for Flavonoids was carried out according to the method of Trease and Evans, 1983 [8].
Test for Tannins was carried out according to the method of Trease and Evans, 1983 [8].
Test for Alkaloids was carried out according to the method of Sofowora, 1982 [9].
Quantitative analysis of phytochemicals
Determination of saponin was carried out according to the gravimetric method of AOAC, 1984 [10].
Determination of total flavonoids was done using the method of Boham and Kocipal-Abyazan [11].
Determination of tannin was done using the standard method described by AOAC [12].
Determination of Glycosides was done using the standard method described by AOAC [10].
Determination of total phenolic contents (TPC) using the Folin- Ciocalteu method adopted by Amin et al. [13] was used.
Ascorbic Acid Contents was determined using the method described by Barros et al. [14].
Determination of Alkaloids was carried out using the procedure described by Harbone (1973) with slight modification by Edeoga et al. [15].
Animals
A total of 54 apparently healthy Wistar albino rats of both sexes weighing between 100-150 g were obtained from the animal house, Department of Pharmacology, Ahmadu Bello University, Zaria, Kaduna State. The animals were separated into male and female in well aerated laboratory cages in the animal house, Department of Pharmacology, Ahmadu Bello University, Zaria, Kaduna State and were allowed to acclimatize to the laboratory environment for a period of two weeks before the commencement of the experiment. They were fed daily with grower mash from Vital Feeds Company and water ad libitum during the stabilization period.
Acute toxicity study
The median lethal dose (LD50) of n-butanol fraction was conducted in order to select a suitable dose for the evaluation of the effects of n-butanol fraction. This was done using the method described by Lorke (1983) [16]. In the initial phase, rats were divided into 3 groups of 3 rats each and were treated with 10 mg, 100 mg and 1000 mg of n-butanol fraction per kg body weight orally. They were observed for 24 hours for signs of toxicity, including death. In the final phase, 3 rats were divided into 3 groups of one rat each, and were treated with n-butanol fraction based on the findings in the first phase. The LD50 was calculated from the results of the final phase as the square root of the product of the lowest lethal dose and the highest non-lethal dose, i.e., the geometric mean of the consecutive doses with 0 and 100% survival rates were recorded.
Animal grouping
A total of 54 Wistar albino rats were used. The rats were divided into carbon tetrachloride induced liver damage group of 6 rats each and LD50 group.
Carbon tetrachloride induced group
Group A: Normal control Rats were given feed and water only. This served as the normal control group (NC).
Group B: Rats were treated with olive oil and served as vehicle control group (VC).
Group C: Rats were treated with 148 mg/kg b.wt. carbon tetrachloride (CCl4) in olive oil. This serves as the CCl4-induced liver damage group (IC).
Group D: Rats were treated with 148 mg/kg b.wt. CCl4 in olive oil+100 mg/kg b.wt. Silymarin as standard drug (CCl4+Std).
Group E: Rats were treated with 148 mg/kg b.wt. CCl4 in olive oil+100 mg/kg b.wt. n-butanol fraction (CCl4+BF).
Group F: Rats were treated with 148 mg/kg b.wt. CCl4 in olive oil + 150 mg/kg b.wt. n-butanol fraction (CCl4+BF).
Group G: Rats were treated with 148 mg/kg b.wt. CCl4 in olive oil + 200 mg/kg b.wt. n-butanol fraction (CCl4+BF).
Induction of liver damage
The liver damage was induced by the administration of carbon tetrachloride (CCl4). Rats were injected intraperitoneally with a single dose of CCl4 (148 mg/kg body weight) as a 1:1 (v/v) solution in olive oil and were fasted for 36 hours before the administration of n- butanol fraction [17]. This was done once a week for a period of four weeks. The administration of n- butanol fraction was done daily by oral intubation for the period of 28 days.
Collection and preparation of sera samples
At the end of 28 days of treatment, the animals were sacrificed by decapitation using chloroform anaesthesia and blood samples were collected from the head wound in plain bottles (for biochemical parameters). The Blood samples collected in plain tubes were allowed to clot and the serum separated by centrifugation using Labofuge 300 centrifuge (Heraeus) at 3000 rpm for 10 minutes and the supernatant (serum) collected was subjected to biochemical screening.
Collection of liver
Immediately after the blood was collected, the liver was quickly excised, trimmed of connective tissues, rinsed with saline to eliminate blood contamination, dried by blotting with filter paper and weighed (so as to calculate the relative weight) and kept on ice. Certain gram of the liver was crushed in 50 mM potassium phosphate buffer (pH 7.4) using mortar and pestle (homogenization) while the rest of the organs were placed in freshly prepared 10% formalin for histopathological studies. It was then centrifuged at 4000 rpm (2700xg) for 15 minutes. Then the supernatant was collected using Pasteur pipette. The percentage change in organ weight of each of the animals was calculated as follows;
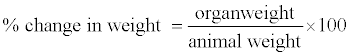
Heamatological assay
Determination of Packed Cell Volume (PCV): The PCV is the volume of red blood cells (RBC) expressed as a fraction of the total volume of the blood. The microhaematocrit method was used.
Biochemical Studies
Assessment of Aspartate Aminotransferase (AST) activity: AST activity was determined by the method described by Amador and Wacker [18].
Assessment of Alanine Aminotransferase (ALT) activity: ALT activity was determined by method described by Amador and Wacker [18].
Assessment of Alkaline Phosphatase (ALP) activity: Serum activity of alkaline phosphatase (ALP) was determined by the method described by Haussament [19].
Determination of Serum Bilirubin Concentration: The serum total and direct bilirubin levels were determined by the method Jendrassik and Gróf [20].
Determination of Total Protein Level: Total protein was determined colorimetrically according to the method described by Fine [21].
Determination of Albumin Level: The serum albumin was determined by the method of Doumas et al. [22].
Estimation of Superoxide Dismutase (SOD) Activity: Superoxide dismutase activity was measured using the method described by Martin et al. [23].
Estimation of Catalase Activity: Catalase activity was determined using the method described by Aebi and Bergmeyer [24].
Estimation of Glutathione Peroxidase: Glutathione peroxidase assay was determined using the method adapted by Paglia and Valentine [25].
Estimation of Thiobarbituric Acid Reactive Substance (TBARS): Thiobarbituric acid reactive substance (TBARS) in the tissues was estimated in the form of MDA using the method described by Fraga et al. [26].
Histopathological studies
A portion of the liver of the animals was cut into two to three pieces and fixed in 10% formalin. The paraffin sections were prepared and stained with haematoxylin and eosin. The thin sections of livers were made into permanent slides and examined under high (X250) resolution microscope with photographic facility and photomicrographs were taken.
Statistical analysis
The data were analyzed by the analysis of variance (ANOVA) using SPSS program (version 17.0 SPSS Inc., Chicago, IL, USA). The differences between the various animal groups were compared using the Duncan Multiple Range Test. The results were expressed as mean ± standard error of mean (SEM). P value less than 0.05 was considered as significant (P<0.05).
The Percentage Yield of Methanolic Leave Extract and Fractions of G. latifolium
The percentage yield (w/w) of the crude extract is (10.24%) and the various fractions have aqueous residue as the highest yield (45.80%), followed by n-butanol fraction (25.14%), ethylacetate fraction (10.70%) and n-hexane fraction has the lowest yield (6.66%).
Lethal Dosage (LD50) determination for n-butanol fraction of G. latifolium
No death was recorded after the oral administration up to a dose of 5000 mg per kg body weight.
Effects of n-butanol fraction of G. latifolium on Packed Cell Volume
The effect of sub-chronic oral administration of n-butanol fraction of G. latifolium methanolic leaves extract and silymarin (Standard drug) at 100 mg/kg b.wt, 150 mg/kg b.wt and 200 mg/kg b.wt. on packed cell volume in CCl4-induced liver damage in albino rats for 28 days is shown in Figure 1. The result showed that the packed cell volume (PCV) level of induced control group was significantly (P<0.05) lowered than the PCV level of normal control group, but there was no significant (P>0.05) difference between the PCV level of the normal control animals and all the induced treated animals.
Figure 1: Mean changes in PCV values of CCl4-induced Liver damage rats treated daily with oral administration of n-butanol fraction of G. latifolium and silymarin (STD).
Values are presented as mean with six replicates for each group.
NC: Normal Control rat, VC: Vehicle control rats, CCl4: Carbon tetrachloride, IC: CCl4 Induced liver damage control rats, CCl4 + BF: CCl4 Induced liver damage rats+100mg/kg b.wt. of n-butanol fraction, CCl4 + BF: CCl4 Induced liver damage rats+150 mg/kg b.wt. of n-butanol fraction, CCl4 + BF: CCl4 Induced liver damage rats+200 mg/kg b.wt. of n-butanol fraction, CCl4 + Std: CCl4 Induced liver damage rats+100 mg/kg b.wt. of Standard Drug (Silymarin).
Effects of n-Butanol fraction of G. latifolium on body and organ weight change
Changes in body weight of rats induced liver damage treated with n-butanol fraction of G. latifolium methanolic leaves extract and silymarin (Standard drug) for a period of 28 days is represented in Figure 2. The results showed no significant (P>0.05) difference in the body weight change of all the induced treated groups compared with the normal control group. However, the CCl4 induced liver damage control group shows a significant (P<0.05) decrease in body weight compared with the induced treated and normal control groups.
Figure 2: Mean Changes in body weights of CCl4-induced Liver damage rats treated daily with oral administration of n-butanol fraction of G. latifolium and silymarin (STD).
Values are presented as mean with six replicates for each group.
NC: Normal Control rat, VC: Vehicle control rats, CCl4: Carbon tetrachloride, IC: CCl4 Induced liver damage control rats, CCl4 + BF: CCl4 Induced liver damage rats+100 mg/kg b.wt. of n-butanol fraction, CCl4 + BF: CCl4 Induced liver damage rats+150 mg/kg b.wt. of n-butanol fraction, CCl4 + BF: CCl4 Induced liver damage rats+200 mg/kg b.wt. of n-butanol fraction, CCl4 + Std: CCl4 Induced liver damage rats+100 mg/kg b.wt of Standard Drug (Silymarin).
Changes in organ weight of rats induced liver damage treated with n-butanol fraction of G. latifolium methanolic leaves extract and silymarin (Standard drug) for a period of 28 days is represented in Table 3. The result showed that there was no significant (P>0.05) difference between the percentage change in liver weights of the entire induced treated group compared with the normal control rats. However, the induced control rats presents a significant (P<0.05) higher percentage change in liver weights compared with the normal control rats.
| Groups (n=6) | % Change in Liver Weight (g) |
|---|---|
| NC | 4.31 ± 0.14a |
| VC | 4.58 ± 0.32a |
| IC | 6.94 ± 0.38b |
| CCl4+ BF | 4.70 ± 0.17a |
| CCl4+ BF | 4.21 ± 0.53a |
| CCl4+ BF | 4.40 ± 0.14a |
| CCl4+ Std | 4.64 ± 0.14a |
Table 3: Mean Changes in Organ Weights of CCl4-Induced Liver Damage Rats Treated Daily with Oral Administration of Silymarin and n-Butanol Fraction of G. latifolium
Values are Means ± SEM. Values with different superscript down the columns are significantly different (P<0.05)
NC: Normal Control rat, VC: Vehicle control rats, CCl4: Carbon tetrachloride, IC: CCl4 Induced liver damage control rats, CCl4 + BF: CCl4 Induced liver damage rats+100mg/kg b.wt. of n-butanol fraction, CCl4 + BF: CCl4 Induced liver damage rats+150mg/kg b.wt. of n-butanol fraction, CCl4 + BF: CCl4 Induced liver damage rats+200mg/kg b.wt. of n-butanol fraction, CCl4 + Std: CCl4 Induced liver damage rats+100mg/kg b.wt. of Standard Drug (Silymarin).
Biochemical studies
Assessment of liver function indices: Liver function indices of alanine aminotransferases (ALT), aspartate amino transferases (AST), alkaline phosphatases (ALP), total protein (TP), albumin (ALB) and bilirubin (DB and IB) concentrations in the serum of CCl4-induced liver and kidney damage rats after the daily oral administration of n-butanol fraction of G. latifolium and silymarin for 28 days is represented in Tables 4 and 5. There was significant (P<0.05) increase in activities of all these liver marker enzymes (ALT, AST and ALP) in the CCl4- induced liver damage control group when compared with the normal control. The activities of ALT, AST and ALP in the induced treated groups were however significantly (P<0.05) reduced when compared with induced not treated group. The n-butanol fraction and silymarin significantly (P<0.05) increase the serum total protein levels of the induced treated groups compared with the induced not treated group but there was no significant (P>0.05) difference between the serum total protein levels of all the induced treated groups and the normal control group. Also serum albumin concentrations of the induced not treated group was significantly (P<0.05) lower than the normal control and the induced treated groups, but there was no significant (P>0.05) difference between the serum albumin levels of all the induced treated groups and the normal control group. Also, the levels of bilirubin in the induced treated groups were however significantly (P<0.05) reduced when compared with induced not treated group, but there was no significant (P>0.05) difference between the bilirubin levels of all the induced treated groups and the normal control group.
| Group (n=6) | ALT (IU/L) | AST (IU/L) | ALP (IU/L) |
|---|---|---|---|
| NC | 45.8 ± 2.85a | 42.5 ± 1.63ab | 60.2 ± 2.18a |
| VC | 44.5 ± 3.24a | 41.3 ± 1.89a | 59.5 ± 1.77a |
| IC | 60.3 ± 3.02b | 56.8 ± 2.18c | 76.0 ± 3.44b |
| CCl4 + BF | 48.0 ± 2.15a | 47.3 ± 1.54b | 64.7 ± 2.33a |
| CCl4 + BF | 47.3 ± 1.63a | 43.8 ± 1.74ab | 62.2 ± 1.11a |
| CCl4 + BF | 45.5 ± 1.73a | 44.5 ± 1.71ab | 61.8 ± 1.78a |
| CCl4 + Std | 48.3 ± 2.03a | 46.7 ± 1.71ab | 64.0 ± 1.81a |
Table 4: Effects of Daily Doses of n-butanol fraction of G. latifolium on Serum Liver Function Parameters (ALT, AST and ALP) of CCl4-Induced Liver Damage Albino Rats.
| Group (n=6) | TP (g/dl) | ALB (g/dl) | DB (mg/dl) | IB (mg/dl) |
|---|---|---|---|---|
| NC | 61.2 ± 1.82cd | 37.2 ± 2.09c | 6.68 ± 0.41a | 5.30 ± 0.69a |
| VC | 60.3 ± 1.94bcd | 33.5 ± 1.52bc | 7.13 ± 0.68a | 5.10 ± 0.23a |
| IC | 44.8 ± 2.21a | 24.8 ± 1.74a | 10.5 ± 0.57b | 6.82 ± 0.24b |
| CCl4 + BF | 55.2 ± 2.02bc | 32.2 ± 1.30bc | 7.70 ± 0.89a | 5.78 ± 0.25ab |
| CCl4 + BF | 59.5 ± 1.48bcd | 29.5 ± 2.49ab | 7.62 ± 0.95a | 4.72 ± 0.24a |
| CCl4 + BF | 63.3 ± 2.06d | 33.3 ± 1.71bc | 6.83 ± 0.78a | 5.55 ± 0.33a |
| CCl4 + Std | 54.5 ± 2.45b | 33.0 ± 1.93bc | 8.23 ± 0.62a | 4.83 ± 0.51a |
Table 5: Effects of Daily Doses of n-butanol fraction of G. latifolium on Serum Total Protein, Albumin, Direct and Indirect Bilirubin of CCl4 Induced Liver Damage Albino Rats.
In vivo antioxidant studies
Effects of n-butanol fraction of G. latifolium on some endogenous antioxidant enzymes in the liver of CCl4-induced liver damage albino rats: The effects of daily oral administration of n-butanol fraction of G. latifolium and Silymarin for 28 days on the level of malondialdehyde (MDA) and some endogenous antioxidant enzymes (catalase, glutathione peroxidase and superoxide dismutase) of the liver of CCl4 induced liver damage rats is represented in Table 6. There was a significant (P<0.05) increase in the level of malondialdehyde (MDA) and a significant (P<0.05) decrease in the level of catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (SOD) of the CCl4 induced liver damage control rats compared with the normal control. There was no significant (P>0.05) difference in the levels of MDA and endogenous antioxidant enzymes of all the induced treated groups when compared with the normal control group.
| Group (n=6) | MDA (μM/ml) | SOD (U/ml) | CAT (U/ml) | GPx (mU/ml) |
|---|---|---|---|---|
| NC | 1.32 ± 0.06a | 2.45 ± 0.08b | 50.0 ± 2.93b | 50.8 ± 1.17b |
| VC | 1.52 ± 0.07a | 2.25 ± 0.08b | 51.2 ± 2.06b | 49.5 ± 1.89b |
| IC | 2.53 ± 0.09b | 1.43 ± 0.09a | 40.3 ± 1.17a | 37.7 ± 1.67a |
| CCl4 + BF | 1.53 ± 0.11a | 2.23 ± 0.10b | 52.0 ± 1.27b | 47.2 ± 1.64b |
| CCl4 + BF | 1.52 ± 0.11a | 2.45 ± 0.09b | 53.0 ± 1.32b | 49.8 ± 1.30b |
| CCl4 + BF | 1.48 ± 0.13a | 2.43 ± 0.09b | 54.5 ± 1.18b | 51.0 ± 1.53b |
| CCl4 + Std | 1.58 ± 0.10a | 2.35 ± 0.11b | 52.5 ± 1.34b | 49.83 ± 2.34b |
Table 6: Effects of Daily Doses of n-butanol fraction of G. latifolium on Some Endogenous Antioxidant Enzymes in the Liver of CCl4-Induced Liver Damage Albino Rats.
Histopathological studies
Effects of n-butanol fraction of G. latifolium on liver: The histological section of the liver of CCl4-induced oxidative damage rats treated with n-butanol fraction of G. latifolium methanolic leave extract and silymarin for 28 days is shown in Plate 1. The histopathological examinations of liver section of normal control group showed normal cellular architecture with distinct hepatic cells. CCl4-induced control group liver showed an intense hepatic necrosis with vascular congestion, vacoulation, lymphocyte hyperplasia and degeneration of normal hepatic cells. The induced treated groups showed almost normalization of the hepatic cells. On daily oral administration of n-butanol fraction of G. latifolium methanolic leave extract and silymarin brought the liver back to moderate hepatic necrosis.
A: Normal hepatocytes
B: Slight vascular congestion
C: Intense vascular congestion, vacoulation, lymphocyte hyperplasia and hepatic necrosis
D: Moderate hepatic necrosis with vacoulation
E: Slight vascular congestion and vacoulation
F: Slight vascular congestion
G: Moderate vascular congestion with slight vacoulation
Plate 1: The Representative Liver Region of CCl4-Induced Liver damage rats treated with n-butanol fraction of G. latifolium and silymarin (H&E STAIN X250).
The preliminary phytochemical studies revealed the presence of glycosides, saponins, tannins, alkaloids, and flavonoids in the crude methanolic leave extracts of G. latifolium. The presence of these phytochemicals in the plant, accounts for its usefulness as medicinal plant [27]. The quantitative phytochemical analysis showed that tannins had the highest concentration in the crude extract (Table 1) whereas the n-butanol fraction had the highest concentration of flavonoids, ascorbic acid and polyphenols [28,29] when compared to the ethylacetate fraction (Table 2). Plant phenolics, flavonoids and ascorbic acid constitute major groups of phytochemicals acting as primary in vitro antioxidants or free radical terminators [30]. Therefore, it was reasonable to determine their concentration in the n-butanol and ethylacetate plant fractions with the aim of utilising the fraction with the highest concentration of in vitro antioxidant [31,32]. Polyphenols, flavonoids and ascorbic acid scavenging potentials and metal chelating ability is [33] dependent upon their unique structure, the number and position of the hydroxyl groups [34-36]. The potential health benefits associated with these phytochemicals has generated great interest among scientists for the development of natural in vitro antioxidant compounds from plants [37,38].
Haematological investigation provides information on the general pathophysiology of the blood and reticuloendothelial system [39,40]. Fairbarks [41] showed that xenobiotics causes low PCV level which may be associated with the oxidization of sulphyldryl groups of the erythrocyte membrane thus, inflicting injury to the erythrocytes membrane. This is in agreement with the present study as packed cells volume (PCV) values in rats exposed to CCl4 gave low levels of PCV. The n-butanol fraction appeared to boost blood cells as the values of PCV approached the normal control (Figure 1). This finding suggests that the administration of the n-butanol fraction of the methanolic leave extract of G. latifolium to patient with remarkable low PCV level may increase their packed cell volume. It implies that the n-butanol fraction may possess constituents that would trigger the production of more blood cells [42,43].
Changes in the body weight after CCl4 dosing have been used as a valuable index of CCl4-related organ damage by [44,45] and thus, will be applicable in this study in order to justify the effects of CCl4 on the body and organ weights of these animals. The decrease in changes in body weight (Figure 2) and consequent increase in liver weights seen in CCl4-induced control group was considered to be as a result of direct toxicity of CCl4 and/or indirect toxicity that lead to liver damage (Table 3). This indicates that CCl4 may have induced hypertrophy of the cells of these organs as well as elicit remarkable tissue damage [46] which may have led to the observed effects on the body and organ weights of these animals. However, all the induced treated groups experienced a significant increase in body weight changes as well as reduced change in organ weights, suggesting the possible curative effects of the n-butanol fraction of G. latifolium against liver injury after CCl4 induction.
Assessment of liver can be made by estimating the activities of serum ALT, AST and ALP which are enzymes originally present at higher concentration in cytoplasm [47]. When there is hepatopathy, these enzymes leak into the blood stream in conformity with the extent of liver damage [48,49]. Administration of CCl4 caused a significant (P<0.05) elevation of these liver marker enzyme levels and a consequent decrease in the level of serum proteins when compared to normal control group (Tables 4 and 5). The elevated level of these marker enzymes with a corresponding decrease in serum proteins level observed in the CCl4-induced not treated group corresponded to the extensive liver damage induced by CCl4 which may lead to an impaired protein turnover. These results are in agreement with previous finding that the activity levels of serum ALT, ALP and AST were significantly elevated as well as a significant decrease in serum protein levels in rats after CCl4 administration [50-53].
Also, the significant (P<0.05) elevation of bilirubin levels in the CCl4-induced not treated group when compared to the normal control and the induced treated groups may be as a result of haemolytic anaemia that may be associated with oxidative damage to red blood cells thus, leading to elevated bilirubin level since bilirubin is an intermediate product in haemoglobin breakdown in the liver [47]. Again, this elevated bilirubin level may also be associated with reduced hepatocyte uptake of bilirubin, impaired conjugation of bilirubin and reduced hepatocyte secretion of bilirubin [48,49]. However, since there are significant elevation of direct (conjugated) and indirect (unconjugated) bilirubin levels in the blood serum of CCl4-induced not treated group, this may be attributed to the inability of the hepatocyte to secrete conjugated bilirubin as envisioned in elevated direct bilirubin level as a result of liver necrosis or may be due to inability of the liver to conjugate bilirubin in the case of elevated indirect bilirubin which can be attributed to the inability of the necrotic liver to conjugate bilirubin or the inability of the hepatocytes to take up bilirubin [50-52]. Also, elevated bilirubin may also be due to obstruction in the flow of bile within the liver or in the bile duct as a result of severe liver damage [43].
There was significant (P<0.05) restoration of these liver marker enzymes activities as well as bilirubin and serum proteins levels on administration of the n-butanol fraction and silymarin for 28 days at a dose of 100 mg/kg b.wt., 150 mg/kg b.wt. and 200 mg/kg b.wt. The reversal of these serum liver marker enzymes in CCl4-induced treated groups towards a near normalcy by the n-butanol fraction observed in this study may be due to the prevention of the leakage of these intracellular enzymes as a result of the presence of polyphenols, flavonoids and ascorbic acid in the n-butanol fraction as well as their membrane stabilizing activity which may be attributed to their ability to mop up free radicals that attack cell membranes. Also, the repeated contact of these in vitro antioxidants with hepatocytes may lead to increased stability of the cell membrane [54]. Again, the ability of the n-butanol fraction to reduce the bilirubin level to near normalcy may be as a result of its ability to assist in the regeneration of the hepatocytes by reducing oxidative damage to red blood cells which may lead to reduction in haemoglobin breakdown by the liver. This is in agreement with the commonly accepted view that serum levels of transaminases, bilirubin and serum proteins returns to normalcy with the healing of hepatic parenchyma cells as well as the regeneration of hepatocytes [14]. It is therefore, a clear manifestation of the hepatocurative effects of the n-butanol fraction of G. latifolium. Following the administration of the n-butanol fraction of G. latifolium and silymarin, the hepatocytes showed close to normal cellular architecture which may be as a result of regeneration and repair of liver cells [43,53].
Antioxidant activity or scavenging activity of the generated free radicals is important in the curative effect of CCl4-induced hepatotoxicity. The body has an effective defence mechanism to prevent and neutralize free radicals-induced damage. This is accomplished by a set of endogeneous antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and catalase. Decrease in enzyme activity of superoxide dismutase (SOD) is a sensitive index in hepatocellular damage and is the most sensitive enzymatic index in liver injury [55,56]. The increased level of malondialdehyde (MDA) in the liver tissue of the rats administered CCl4 (Table 6) may be as a result of the enhanced membrane lipid peroxidation by free radicals generated and failure of antioxidant defence mechanisms to prevent formation of excessive free radicals [50,57,58]. Also, the decreased activity of SOD, GPx and CAT in the liver tissues of CCl4-induced rats may be due to high concentration of these free radicals generated by CCl4 which may lead to decreased level or inactivation of these endogenous antioxidant enzymes [59]. Treatment with n-butanol fraction of G. latifolium significantly (P<0.05) increased the levels of SOD, GPx and CAT activities and a consequent significant (P<0.05) reduction in MDA. The effects of the n-butanol fraction were comparable to the standard drug (Silymarin). Thus, this result suggests that n-butanol fraction of G. latifolium contains free radical scavenging activity due to the presence of in vitro antioxidants, which could exert beneficial action against pathophysiological alterations caused by the presence of superoxide and hydroxide free radicals as well as hydrogen peroxide indicating the regeneration of damaged liver cells [2,53].
The histopathological studies of the liver in the CCl4-induced control group showed that CCl4 caused an intense vascular congestion, vacoulation, lymphocyte hyperplasia and necrosis (Plate 1) indicating its hepatotoxicity. This result is in agreement with [60]. Following the administration of the n-butanol fraction of G. latifolium and silymarin, the hepatocytes showed close to normal cellular architecture which may be as a result of regeneration and repair of liver cells [43,53]. In line with these findings, it’s obvious that histopathological examinations are in agreement with observed biochemical analysis. This result is in agreement with the report [2,53,61-68].
The result of this study has scientifically justified the traditional use of G. latifolium in the management of human diseases. The result showed that the n-butanol fraction of methanolic leave extract of G. latifolium possess in vitro antioxidants which may have contributed to its significant anti-hepatotoxic properties. The histological examination showed that the n-butanol fraction of G. latifolium has curative effect on the liver in CCl4-induced liver damage rats. The n-butanol fraction of G. latifolium is comparable to the standard drug (silymarin). This work provides the phytotherapeutic potential of n-butanol fraction of G. latifolium that may be useful to scientists and researchers in the nutraceutical industry.
1. There is need to carry out a bioactivity-guided fractionation, isolation and identification of the bioactive constituents of the n-butanol fraction which is responsible for the observed pharmacological activities.
2. There is need to carry out chronic toxicity studies of the n-butanol fraction of the plant so as to ascertain the safety of long term usage on animals.