Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Research Article - (2015) Volume 5, Issue 3
Objectives: To investigate remission rates in rheumatoid arthritis patients exposed to tocilizumab treatment in real life clinical practice and to test whether concomitant conventional and previous biological disease modifying anti-rheumatic drugs treatment affects the efficacy of tocilizumab to reach remission.
Methods: Between January 2009 and December 2012 disease activity was analyzed in 272 rheumatoid arthritis patients exposed to tocilizumab at the onset of treatment and sequentially thereafter, i.e. every four weeks at the time of infusion. Aside from demographic and disease-specific variables, previous and concomitant conventional and biologic disease modifying anti-rheumatic drugs therapy was documented in all patients. Multivariate logistic regression analyses were conducted to identify factors influencing Disease Activity Score 28 remission and attrition to tocilizumab treatment.
Results: 219 (80.5%) of all patients were female. Mean Age was 55.48 ± 13.23 years and our patients had mean disease duration of 12.48 ± 9.30 years. Disease Activity Score 28 significantly decreased from 5.00 ± 1.52 at baseline to 3.09 ± 1.49 at the latest infusion. Mean treatment period was 58.28 ± 43.95 weeks. A total of 101 patients (42.8%) achieved Disease Activity Score 28 remission, which was significantly associated to the length of tocilizumab exposure. Achievement of Disease Activity Score 28 remission was independent from the concomitant use of conventional disease modifying anti-rheumatic drugs. Previous exposure to tumour necrosis factor inhibitors but not rituximab significantly reduced the likelihood to achieve Disease Activity Score 28 remission. Two logistic regression analyses revealed baseline disease activity and duration of tocilizumab therapy as independent factors for Disease Activity Score 28 remission, whereas age and concomitant methotrexate therapy were linked to attrition to tocilizumab treatment.
Conclusion: Remission rates found in this observational study are comparable to those of randomized controlled trials and those of big post-marketing surveillance studies. Remission rates of rheumatoid arthritis patients treated with tocilizumab in clinical practice are not influenced by concomitant disease modifying anti-rheumatic drugs use. Previous exposure to tumour necrosis factor inhibitors but not to rituximab decreases the chance to reach remission with tocilizumab.
<Keywords: Tocilizumab; Remission; DMARD; MTX; Predictors of response; Drug attrition; Real life
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that affects about 0.6% of the adult population and is associated with substantial impairment of function and reduced quality of life [1]. RA is characterized by synovial inflammation with joint swelling and pain leading to destruction of cartilage and bone. Over the past years the importance of different cytokines in the pathogenesis of RA has been discovered and their specific therapeutic neutralization by antibodies or soluble receptors has crucially improved the treatment and outcome of RA patients [2].
Next to tumour necrosis factor alpha (TNF), interleukin (IL)-6 is a key cytokine involved in the pathogenesis of RA [3]. Physiologically, IL-6 is expressed in case of stress associated with conditions such as infections, and production ceases when these stress factors disappear. IL-6 plays a decisive role in B- and T-cell-differentiation, in activation of acute-phase-protein-synthesis, in activation of osteoclasts and regulation of other metabolic processes associated with inflammation but also associated with autoimmune diseases. A continuing, deregulated expression of IL-6 has been discovered to be an important factor in the pathogenesis of RA [4].
Tocilizumab (TCZ), a humanized monoclonal antibody blocks IL-6 function through binding to both the soluble and membrane-bound IL-6 receptors [5]. Today TCZ represents an important part in the therapy of RA, indicated by the 2013 update of the European League Against Rheumatism (EULAR) recommendations for the management of RA, where TNF-inhibitors, TCZ and abatacept are recommended equally for the treatment of disease modifying anti-rheumatic drug (DMARD) resistant RA [6]. Numerous randomized controlled trials of TCZ confirming its efficacy have been conducted so far, but only few data are available on TCZ in daily clinical practice [7]. Randomized controlled trials cannot fully reflect the real-life clinical practice as they follow a strictly defined treatment protocol and only a fraction of patients actually treated with biological DMARDs would meet the inclusion criteria for the major randomized controlled trials [8]. Hence we retrospectively analysed the data of 272 TCZ-treated RA patients from eight outpatient centres in Germany to investigate remission rates in RA patients exposed to TCZ treatment in real life clinical practice and to test whether concomitant conventional and previous biological DMARD treatment affects the efficacy of TCZ to reach remission.
Patients
We retrospectively analyzed the data of 272 RA patients collected during the period of TCZ admission to the European market (January 2009 and December 2012) [9]. All data, including information on demographic background and treatment history, were collected from medical records. The study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board.
Patients underwent TCZ treatment in one of eight outpatient centres in Southern Germany. All patients fulfilled the revised 1987 American College of Rheumatology (ACR) criteria for RA [10], were at least 18 years old and received TCZ in the recommended dose of 8mg/kg intravenously every 4 weeks [11]. Changes of treatment intervals were allowed, e.g. in case of adverse events or according to local guidelines or decision by the responsible rheumatologist.
Information on medical and demographic background was taken from the patients’ records. In order to capture the real clinical treatment situation, no limitations with respect to concomitant classical DMARD-therapy were made. Biological DMARD-therapy had to be ceased before initiation of TCZ therapy. In order to assess disease activity and therapeutic response, we evaluated the four variable Disease Activity Score 28 (DAS28) including erythrocyte sedimentation rate (ESR) at the day of the first and the day of the latest infusion. The corresponding scores were additionally classified into the categories of disease activity, i.e. DAS28<2.6: remission, DAS28 ≥ 2.6 and ≤ 3.2: low disease activity; DAS28>3.2 and ≤ 5.1: moderate disease activity and DAS28>5.1: high disease activity [12].
Statistical data analysis
The statistical data analysis comprised descriptive as well as inferential elements, whereas the first characteristics were supposed to reflect the clinical and demographic information of our patients at initiation of TCZ treatment in view of age, sex, disease duration, previous and concomitant anti-rheumatic therapies as well as current disease activity. For nominal data (i.e. sex, seropositivity in view of rheumatoid factor and anti citrullinated peptide antibodies (ACPA), frequency of previous or current antirheumatic therapies categorized by drug), information on frequency is provided while for metric data (i.e. age, disease duration, DAS28-ESR, number of previous DMARDs), arithmetic means, standard deviations, and 95% confidence intervals of the mean are presented for each characteristic. To identify efficacy of TCZ in real life clinical-practice we assessed percentage of patients achieving DAS28-remission. Subsequently further analyses were carried out: The inferential part of the data analysis comprised a dependent samples t-test on disease activity and χ2 tests in order to investigate the relation of DAS28-remission (at the latest infusion) to common previous DMARD-therapies, mode of TCZ-therapy and TCZ-treatment period. In the final step of the analysis, we used two logistic regression models to investigate relations in a multivariate setting: The first model was supposed to identify potential predictors of DAS28-remission at the latest infusion from baseline characteristics whereas the goal of the second model was to investigate predictors of TCZ persistence defined as treatment duration of more than 52 weeks. Both models used an enter procedure in order to include all chosen predictors into analysis and comprised the following set of independent variables: Age, sex, disease duration, rheumatoid factor (RF) and positivity for ACPA, DAS28 at initiation of TCZ-therapy, number of previous therapies, concomitant methotrexate (MTX) and/or glucocorticoid treatment, and whether TCZ-treatment was stopped due to any reasons yet. The regression model for DAS28 remission comprised the duration of TCZ-treatment as an additional predictor. Statistical analysis was done using IBM SPSS Statistics Version 21. All inferential tests were computed using two-tailed testing procedures. Corresponding statistical test assumptions were checked before test computation and are discussed if necessary.
Patients’ characteristics
Data of 272 RA patients (53 male and 219 female) were analysed. Detailed information on their baseline characteristics are shown in Table 1. Briefly, mean age was 55.48 ± 13.23 years with mean disease duration of 12.48 ± 9.30 years. Information from routine lab tests indicated that 185 patients were RF positive and 165 ACPA positive, respectively. Almost all patients (96.7%, 263 patients) had a history of conventional DMARD-treatment with a mean number of 2 previous DMARDs. 216 patients (79.4%) had previously been treated with TNF-Inhibitors, while 74 (27.2%) had a prior use of rituximab (RTX). Furthermore, 222 patients (82.5%) were using concomitant glucocorticoids and 132 patients (48.5%) were using at least one concomitant DMARD. Hence 140 patients (51.5%) were treated with TCZ monotherapy. All patients receiving TCZ-monotherapy had a history of conventional or biological DMARD treatment. Mean of follow-up time/duration of TCZ-treatment was slightly more than one year (58.28 ± 43.95 weeks) and ranged from 4 to 153 weeks.
| N | Min. | Max. | Mean | SD | 95%CI of mean | |
|---|---|---|---|---|---|---|
| Patients | 272 | |||||
| Sex (N male/N female) | 272 | 53 (19.5%) / 219 (80.5%) | ||||
| Age (years) | 272 | 19 | 87 | 55.48 | 13.23 | [53.90; 57.06] |
| RA duration (years) | 265 | 1,17 | 45,00 | 12.48 | 9.30 | [11.36; 13.61] |
| Rheumatoid factor positive (N positive/N negative) |
262 | 185 (70.6%) / 77 (29.4%) | ||||
| ACPA positive (N positive/N negative) |
254 | 165 (65.0%) / 89 (35.0%) | ||||
| DAS28-ESR | 185 | 0.96 | 8.80 | 5.00 | 1.52 | [4.78; 5.22] |
| Previous therapies | ||||||
| No. of previous DMARDs | 272 | 0 | 7 | 2.18 | 1.094 | [2.05; 2.31] |
| Prior use of TNFα-Inhibitors (N yes/N no) |
272 | 216 (79.4%) / 56 (20.6%) | ||||
| Prior use of Rituximab (N yes/N no) |
272 | 74 (27.2%) / 198 (72.8%) | ||||
| Concomitant therapy | ||||||
| Steroid (N yes/N no) |
269 | 222 (82.5%) / 47 (17.5%) | ||||
| MTX (N yes/N no) |
271 | 106 (39.1%) / 165 (60.9%) | ||||
| Any DMARD (including MTX) (N yes/N no) |
272 | 132 (48.5%) / 140 (51.5%) | ||||
Table 1: Descriptive cohort characteristics at baseline./p>
| B | S.E. | Wald | df | p-value | OR | 95% CI (OR) | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Age | -0.01 | 0.02 | 0.46 | 1 | 0.498 | 0.99 | 0.96 | 1.02 |
| Sex | -0.11 | 0.44 | 0.06 | 1 | 0.801 | 0.89 | 0.38 | 2.13 |
| Disease duration | 0 | 0 | 0.48 | 1 | 0.488 | 1 | 1 | 1 |
| RF | -0.08 | 0.72 | 0.01 | 1 | 0.916 | 0.93 | 0.23 | 3.8 |
| CCP | 0.25 | 0.68 | 0.13 | 1 | 0.714 | 1.28 | 0.34 | 4.87 |
| Baseline DAS28 | -0.32 | 0.13 | 5.67 | 1 | 0.017 | 0.73 | 0.56 | 0.94 |
| Number of previous therapies | -0.22 | 0.13 | 3.17 | 1 | 0.075 | 0.8 | 0.62 | 1.02 |
| Concomitant MTX | 0.03 | 0.38 | 0.01 | 1 | 0.944 | 1.03 | 0.49 | 2.16 |
| Concomitant glucocorticoids | 0.02 | 0.53 | 0 | 1 | 0.976 | 1.02 | 0.36 | 2.84 |
| Therapy with Tocilizumab stopped | -0.49 | 0.48 | 1.03 | 1 | 0.31 | 0.61 | 0.24 | 1.57 |
| Duration of Tocilizumab therapy (weeks) | 0.02 | 0.01 | 9.49 | 1 | 0.002 | 1.02 | 1.01 | 1.03 |
| Intercept | 2.11 | 1.3 | 2.61 | 1 | 0.107 | 8.21 | ||
This logistic regression shows predictors of DAS28-remission at latest infusion. All variables were entered on a single step. B=Unstandardized regression coefficient; S.E.=Standard Error; df=Degrees of Freedom; OR=Odds Ratio; CI (OR)=Confidence Interval (odds ratio)
Table 2: Multivariate logistic regression model for parameters predictive of DAS28-remission.
Effects of TCZ on disease activity of RA
At baseline patients showed moderate to high disease activity according to the DAS28 score (mean DAS28: 5.00 ± 1.52) with significant improvement at the end of the observation period (ΔDAS28: 2.09 ± 1.74; t(172)=15.82, p<0.001) [12]. At that time, low disease activity (DAS28 ≤ 3.2) was observed in 134 patients (56.8%) while among these 101 patients (42.8%) were in DAS28-remission. With patients being included continuously between January 2009 and December 2012 and pursued until the last registered visit before 31st of December 2012, observation periods differed in their duration.
Also cessation of treatment due to various reasons, e.g. adverse events, contributed to that fact. Hence, we divided the cohort into four groups, according to the duration of their treatment period: Group 1 (observation period 4-12 weeks), group 2 (13-24 weeks), group 3(25-52 weeks), and group 4 (>52 weeks). Remission rates were as low as 17.0% (8 patients) in group 1, but 30.8% (8 patients), 34.1% (14 patients) and 58.2% (71 patients) in groups 2, 3 and 4, respectively (Figure 1). A corresponding 2 x 4 Table-χ2 test indicated that remission and the duration of TCZ-therapy according to the aforementioned groups are significantly related to each other χ2(3)=27.36, p<0.001.
Efficacy of TCZ with or without concomitant DMARD therapy
We also investigated whether concomitant conventional DMARDs, i.e. MTX, have an impact on the therapeutic response to TCZ. We therefore compared the efficacy of monotherapy with TCZ with the combination of TCZ and concomitant conventional DMARDs (Figure 2).
The corresponding results suggested, that DAS28 remission is independent from the concomitant use of conventional DMARDs χ2(1)=0.92, p=0.359. Additionally we did not see any difference between the two groups when comparing the remission rates according to the above mentioned treatment periods.
Efficacy of TCZ after failure of biological DMARDs
We next evaluated whether previous use of other biological DMARDs influenced the achievement of DAS28 remission in TCZ treated patients. Whereas previous RTX treatment did not influence the remission rate achieved with TCZ (χ2(1)=2.56, p=0.115), previous TNF-inhibitor treatment significantly reduced the likelihood of TCZ to reach remission (χ2(1)=7.67, p=0.006). Hence only 38.17% of the RA patients having been exposed to TNF-inhibitors compared to 60% of RA patient’s naïve for TNFα inhibitor reached clinical remission with TCZ.
Predictors of DAS28-remission
A logistic regression for DAS28-remission at the time of the latest infusion showed a correct case classification rate of 65.1% (Table 2). This model suggested the DAS28 at start of TCZ-treatment (Wald coefficient=5.67, p=0.017) and the period of TCZ-treatment (Wald coefficient=9.49, p=0.002) to be independent predictors of later DAS28-remission (Table 2). From these predictors, only the latter was positively associated with remission, highlighting that longer TCZ-treatment period makes achievement of remission more likely. Higher DAS28 scores at start of therapy seem to decrease the likelihood of subsequent remission. Demographic variables as well as common concomitant therapies such as MTX or glucocorticoids were not related to the outcome of interest in this model.
Attrition to TCZ therapy
The mean duration of TCZ treatment in this cohort was cohort was 58.3 ± 44.0 weeks. Overall, 77 patients (28.3%) withdrew from TCZ treatment, most of them because of lack of efficacy (37 patients, 13.6%). 15 patients (5.5%) stopped for reason of adverse events in general, 10 patients (3.7%) due to infections (i.e. altogether 25 patients (9.2%) due to adverse events) and 15 patients (5.5%) had other reasons to stop treatment, for example, in case of patients wishing to become pregnant. 125 patients (47.9%) received TCZ at least 53 weeks and thus were defined being drug persistent. Performing a multivariate logistic regression analysis we identified independent predictors of drug persistence (Table 3).
In the corresponding regression model (correct case classification rate: 72.5%,), age (Wald coefficient=5.76, p=0.016), concomitant therapy with MTX (Wald χ2=4.39, p=0.036) and the stop of TCZ-treatment due to one of the aforementioned reasons (Wald coefficient=24.76, p<0.001) were independently related to drug persistence with TCZ (Table 3).According to the findings of this model, events leading to a stop of TCZ-therapy were more likely to occur in the first 52 weeks of therapy whereas patients of higher age and patients concomitantly treated with MTX were more likely to be drug persistent. Other demographic or disease-related characteristics did not seem to be of relevance in this regression model.
| B | S.E. | Wald | df | p-value | OR | 95% CI (OR) | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Age | 0.04 | 0.02 | 5.76 | 1 | 0.016 | 1.04 | 1.01 | 1.07 |
| Sex | 0.67 | 0.47 | 2.06 | 1 | 0.151 | 1.96 | 0.78 | 4.92 |
| Disease duration | 0 | 0 | 2.75 | 1 | 0.098 | 1 | 1 | 1.01 |
| RF | -0.07 | 0.78 | 0.01 | 1 | 0.924 | 0.93 | 0.2 | 4.3 |
| CCP | -0.27 | 0.75 | 0.13 | 1 | 0.715 | 0.76 | 0.17 | 3.31 |
| Baseline DAS28 | -0.17 | 0.13 | 1.59 | 1 | 0.208 | 0.84 | 0.65 | 1.1 |
| Number of previous therapies | 0.1 | 0.12 | 0.73 | 1 | 0.394 | 1.11 | 0.88 | 1.39 |
| Concomitant MTX | 0.82 | 0.39 | 4.39 | 1 | 0.036 | 2.26 | 1.05 | 4.87 |
| Concomitant glucocorticoids | 0.53 | 0.54 | 0.96 | 1 | 0.327 | 1.69 | 0.59 | 4.86 |
| Therapy with Tocilizumab stopped | -2.55 | 0.51 | 24.76 | 1 | <0.001 | 0.08 | 0.03 | 0.21 |
| Intercept | -2.01 | 1.23 | 2.68 | 1 | 0.101 | 0.13 | ||
This logistic regression shows predictors of DAS28-remission at latest infusion. All variables were entered on a single step. B=Unstandardized regression coefficient; SE: Standard Error; df: Degrees of Freedom; OR: Odds Ratio; CI (OR): Confidence Interval (Odds Ratio)
Table 3: Multivariate logistic regression model for parameters predictive of drug persistence (i.e. treatment period>52 weeks).
This study in 272 RA-patients was performed to provide further data on the efficacy of the interleukin-6 receptor inhibitor TCZ and to identify factors for efficacious and long lasting therapy in routine treatment of RA. After an average treatment period of 58.28 weeks, 42.8% of patients achieved DAS28-remission and 56.8% had low disease activity (DAS28 ≤ 3.2). These results are in accordance with efficacy data collected in the REACTION study on 232 RA patients that revealed a comparable remission rate of 43.7% at week 52 [13] or the Japanese post-marketing surveillance of 7901 patients with a DAS28-remission rate of 47.6% at week 28 [14].
When addressing remission rates according to the duration of therapy, we found 30.8% in remission after 13 to 24 weeks exposure to TCZ. This result is fairly similar to the results of the phase III randomized controlled trials of TCZ that predominantly had an observation period of 24 weeks [15-21]. Remission rates in these studies ranged from 27% in the OPTION study [16] to 59% in the SAMURAI study [20]. Patients exposed to TCZ between 25 and 52 weeks (34.1%) or those receiving treatment for more than 52 weeks (58.2%) showed higher prevalence of remission, which can be interpreted as a continuous improvement in efficacy with increasing time of exposure to TCZ as previously reported by Genovese et al. in a long-term safety and efficacy analysis of TCZ [22]. Furthermore, the proportion of patients that stopped TCZ treatment due to inadequate response was comparable in patients exposed less than 12 weeks (19.6%), 13-24 weeks (24.1%) and 24-52 weeks (24.1%), whereas it was very low (3.8%) in those treated for more than 52 weeks. Hence there is a selection for well-responding patients in those treated with TCZ for more than 52 weeks explaining the high prevalence of remission.
Furthermore we discovered, that the baseline DAS28 is related to remission with lower baseline values indicating a higher likelihood of achieving later remission-this is in accordance with numerous findings in literature [13,14,23]. In their summary of postmarketing surveillance Koike and colleagues defined less advanced RA (Steinbrocker class 1+2), no previous exposure to biologics and lower baseline DAS28 as predictive factors for DAS28-remission [14]. In accordance with these findings, the number of previous therapies showed a trend for predicting the likelihood for remission but failed to reach significance with higher numbers of previous DMARDs reducing the chances for reaching remission.
Even though biological DMARDs are recommended to be administered in combination with MTX or other conventional DMARDs according to the EULAR recommendations 2010 and 2013 update [6,24], recent data show that about one third of patients are treated with TCZ monotherapy [25-27]. In our cohort even 51.5% (140) of all patients were administered monotherapy. TCZ monotherapy has shown greater efficacy than MTX or DMARD in several studies, such as AMBITION, SAMURAI, or the SATORI study [15,20,21]. Moreover, results of the ADACTA-study, published in 2013, indicate that TCZ monotherapy is more effective in reducing signs and symptoms of RA than the adalimumab monotherapy [28]. By contrast, results of both randomized controlled trials and observational studies comparing TCZ monotherapy and TCZ with concomitant MTX or DMARDs are quite controversial. The 52-week REACTION study for example revealed better outcome in patients receiving concomitant MTX [13] in opposition to the findings in French clinical practice data [25] that did not demonstrate any significant difference between patients with or without concomitant MTX. Furthermore, the 24-weeks ACT-RAY randomized controlled trial did not show any difference in the outcome between the two groups of patients [29]. The recently published 1-year results of the ACT-RAY study did not demonstrate any difference either, although a trend towards a benefit of the add-on strategy was found for some individual parameters [30]. In this cohort, concomitant MTX therapy was not associated to a higher likelihood to reach DAS28 remission. Hence, TCZ monotherapy might be considered an effective treatment option in daily clinical practice as no differences between monotherapy and therapy with concomitant MTX were found [31]. Nonetheless, concomitant MTX was found being associated with better attrition to TCZ, however, these conclusions should be drawn with care as we do not know the reasons why individual patients are treated with mono- or combination therapy.
Comparing the outcome of TNF-inhibitor-naïve and TNF-inhibitor-pretreated patients, we detected significant higher DAS28-remission rates in the anti-TNF-naïve group (DAS28-ESR remission rate: 60.0% vs. 38.2%). Although approved for that indication, TCZ had not been recommended as first choice treatment for DMARD inadequate responders by the EULAR until the update of the recommendation in 2013 [6,24]. That’s reflected in a rate of 79.4% of patients in our cohort being pretreated with at least one TNF-inhibitor. Taking into account that in our cohort patients with previous TNF-inhibitor therapy had significantly longer disease duration and a significantly higher number of previous DMARD-therapies, we assume that the difference in treatment response between TNF-inhibitor-naïve and TNF-inhibitor-pretreated patients is related to a more longstanding and refractory type of RA in patients previously treated with TNF-inhibitors. The TAMARA-study, the ACT-SURE-study or the retrospective observational study of Wakabayashi et al. for example show similar results [32-34].
Prior treatment with RTX, a B-cell-depleting biological DMARD [35], was a criterion for exclusion in several randomized clinical trials of TCZ, like for example the TOWARD- or the TAMARA-study [17,32]. Seeing that past RTX therapy is quite common in real-life medical care (27.2% of patients in our cohort) remission rates of patients with and without previous rituximab therapy was compared. However, different from previous TNF-inhibitor-treatment, no significant difference in remission rates was detected whether patients received previous RTX treatment or not, although also those patients with previous RTX had significantly longer disease duration than those without such pre-treatments.
Limitations of this study arise from its retrospective, observational character. As mentioned, bias by selection of well responding patients cannot be fully excluded, although data from all patients receiving TCZ were included irrespective how long they were exposed to the drug. In addition retrospective data collection has resulted in missing data. Furthermore our study was not blinded, which might have resulted in bias concerning evaluation of efficacy. Another limitation was the potential lack of linearity of the logit that we found in the regression diagnostic of the models. However, we felt that a mathematical transformation of our data would result in a loss of comprehensiveness of the variables for the interested reader and therefore favoured leaving them unchanged. We also realized that including rheumatoid factor and ACPA into the same regression model might result in multicollinear tendencies for these predictors - although this approach is reasonable from a rheumatologic point of view. Furthermore, there might be additional characteristics that have not been incorporated yet such as psychological status which might potentially influence drug persistence or even attainment of DAS28 remission and that might be capable of further enhancing correct case classification by means of logistic regression. Still our data provide relevant information as they well reflect the real-life situation of treatment with TCZ rather than the highly regulated and somewhat artificial conditions in randomized controlled trials.
In summary, these data show that efficacy of TCZ treatment in real-life clinical practice is comparable to that found in randomized controlled trials and big post-marketing surveillance studies. Furthermore factors related to remission and drug attrition were defined. Additionally these data show that TCZ monotherapy is not inferior to its combination with conventional DMARDs and that previous exposure to TNF-inhibitors but not to RTX decreases the chance to reach remission with TCZ in real-life clinical practice.
To assess disease activity and therapeutic response, we used the four variable Disease Activity Score 28 (DAS28) including erythrocyte sedimentation rate (ESR). This summation score includes a tender joint count (TJC; 0-28; proximal interphalangeal joints, metacarpophalangeal joints, wrists, elbows, shoulders, knees), a swollen joint count (SJC; 0-28; proximal interphalangeal joints, metacarpophalangeal joints, wrists, elbows, shoulders, knees), ESR (mm/h) and patient’s global health rating (Patient Global; 0-100 mm on visual analogue scale) [36-38]:
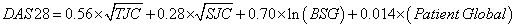
This work was supported by the Deutsche Forschungsgemeinschaft (SPP1468-IMMUNOBONE), the Bundesministerium für Bildung und Forschung (BMBF; project ANCYLOSS), the MASTERSWITCH and TEAM project of the European Union and the IMI funded project BTCure. The present work was performed by the MD student Kathrin Standfest (Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU)) in fulfilment of the requirements for obtaining the degree “Dr. med”.
K.S., K.K., W.O., H.K., J.W., H.S.-K., M.W., J.Z., AJ.H. have nothing to disclose. M.E. reports personal fees from Chugai, grants and personal fees from Roche outside the submitted work. M.S.-H. reports personal fees from Abbvie, Pfizer, Roche. H.-P. T. reports personal fees from Roche, Chugai, Abbvie, MSD; J.Z. reports personal fees from Roche, MSD, BMS, Pfizer, Abbvie, Novartis, GSK, UCB, Astro Pharma outside the submitted work. S.K. reports personal fees from Chugai Pharma Ltd. during the conduct of the study. M.F. reports personal fees from Roche, Chugai, Pfizer, Abbvie, MSD outside the submitted work. F.S. reports personal fees from Roche, Abbvie, MSD, personal fees and other from Novartis, other from Sanofi Aventis outside the submitted work. M.G. reports personal fees from Roche, MSD, BMS, Pfizer, Abbvie, Novartis, GSK, UCB outside the submitted work. M.F. reports other from Roche, Chugai outside the submitted work. K.M. reports personal fees from Pfizer, Abbvie outside the submitted work. B.M. reports personal fees from Roche, Abbvie, MSD, Novartis, SOBI, UCB, BMS, Pfizer, Chugai, Janssen, Astra-Zeneca outside the submitted work. G.S. reports personal fees from Roche, Abbvie, MSD, Celgene, Novartis outside the submitted work. J.R. reports personal fees from Roche, MSD, BMS, Pfizer, Abbvie, Novartis, GSK, UCB outside the submitted work.