Rheumatology: Current Research
Open Access
ISSN: 2161-1149 (Printed)
ISSN: 2161-1149 (Printed)
Review Article - (2021)Volume 11, Issue 4
Rheumatoid Arthritis (RA) is associated with an increased risk of Serious Infection Events (SIE), lymphomas and with increased death rates due to cardiovascular disease. The main objective of this review analysis is to find the Effectiveness and safety of rituximab in patients with active rheumatoid arthritis. This study was conducted using a systematic search on Google scholar, PubMed and Web of science published until 20th April 2021. The cited references of retrieved articles and previous reviews were also manually checked to identify any additional eligible studies. There were total 188 studies that identified initially from PubMed, Google scholar and Web of Science databases. After excluding duplicates and articles that did not meet the inclusion criteria, we obtained 90 articles with full-texts which were read for further evaluation, where another 75 were excluded as irrelevant. Overall, we included 15 articles that directly match on the inclusion criteria. It is concluded that rituximab is effective primarily in seropositive RA. Recent studies have further supported the efficacy of reduced dosage and different regimens, although more work is needed to establish the optimal strategy. Safety data from rheumatology as well as oncology literature highlight the need for hepatitis screening as well as checking pre-treatment immunoglobulin levels to identify patients possibly at greater risk of infection. Data thus far do not indicate the need for routine tuberculosis screening.
B-lymphocyte cells; Sjogren's syndrome; Methotrexate; Lupus erythematous
Rheumatoid arthritis (RA) is associated with an increased risk of Serious Infection Events (SIE), lymphomas and with increased death rates due to cardiovascular disease. Biological Disease- Modifying Antirheumatic drugs (DMARDs), including Tumour Necrosis Factor (TNF) inhibitors, may further increase the risk of SIE and malignancies. Continual monitoring of long-term safety of patients receiving biological therapies is therefore of paramount importance [1]. Although cytokine-specific biologic agents have improved the treatment of Rheumatoid Arthritis (RA), some patients have an inadequate response to such therapies and, in clinical practice, most have only a partial response. The frequency of remission has remained <50% for patients with established RA, many of whom display “moderate” disease activity, according to their Disease Activity Score (DAS). In order to achieve additional significant gains in RA therapy, new targets need to be identified. One such target is the B cell [2]. Rituximab is a monoclonal antibody that selectively depletes CD20+ B cells via 3 putative mechanisms: antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and promotion of CD20+ B cell apoptosis. CD20 is not expressed on stem cells or plasma cells; this is consistent with the observation that B cell recovery and Ig production were not compromised after a single course of rituximab [3]. B celltargeted therapy using the anti-CD20 monoclonal antibody rituximab is an effective treatment for RA. In combination with Methotrexate (MTX), rituximab improves the signs and symptoms of RA and slows joint damage progression [4]. Data from eight randomised, placebo-controlled trials in patients with moderately to severely active RA demonstrated that the overall rates of Adverse Events (AEs) and Serious Adverse Events (SAEs), including SIE, were similar to those observed with placebo+ MTX. Long-term follow-up of patients in these trials indicated that rituximab remained well tolerated over time and multiple courses [5]. These data are encouraging; however, the safety of repeated peripheral B cell depletion, in particular regarding the potential cumulative risk of SIE and malignancies, remains to be fully established [6].
Rituximab (RTX) was approved in Spain for the treatment of lymphoma in June 1998 and Rheumatoid Arthritis (RA) in June 2006. Research in RA was initially based on evidence of efficacy and safety of the drug in patients with lymphoma, which has a particular clinical development [7]. The first detailed clinical data on the use of RTX in RA was published in 2001 and included: patients with no prior exposure to Methotrexate (MTX), patients that failed MTX and/or other disease-modifying drugs (DMARDs), and patients failing treatment with drugs that block Tumour Necrosis Factor (TNF) alpha (anti-TNF-α) [8]. Currently, there are more than 7 years of experience with the use of RTX in patients with RA and Spain has an increasingly larger number of patients with RTX, not only with RA but patients with severe and refractory manifestations of lupus erythematous, vasculitis, Sjogren's syndrome, and other indications, showing good results [8].
Objective
The main objective of this review analysis is to find the Effectiveness and safety of rituximab in patients with active rheumatoid arthritis.
This study was conducted using a systematic search on Google scholar, Pubmed and Web of science published until 20 th April 2021. The cited references of retrieved articles and previous reviews were also manually checked to identify any additional eligible studies.
Data collection
All citations were imported into a bibliographic database and duplicates were removed. Title, abstract and then full-text of all articles were screened for eligibility. The search strategy was similar for all bases: MESH terms and free text for the following terms: rituximab, rheumatoid arthritis, anti-CD20, and biologics; we did not select any publication language filter or type of study. Collaboration tool for data extraction, which includes information on the type of clinical trial, patient characteristics, number of centers, type of intervention, efficacy and safety outcomes, and features analysis as shown in Figure 1.
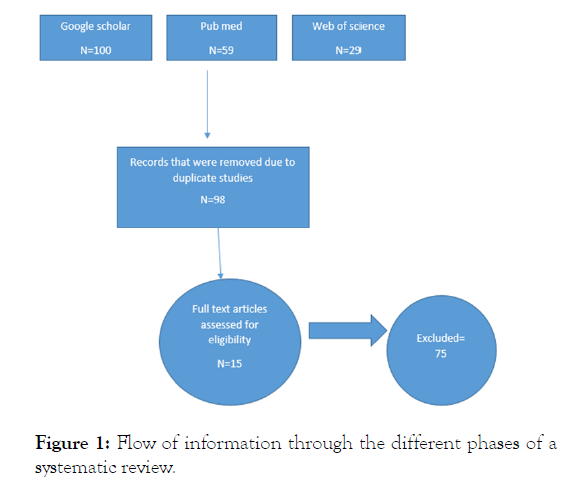
Figure 1: Flow of information through the different phases of a systematic review.
There were total 188 studies that identified initially from PubMed, Google scholar and Web of Science databases. After excluding duplicates and articles that did not meet the inclusion criteria, we obtained 90 articles with full-texts which were read for further evaluation, where another 75 were excluded as irrelevant. Overall, we included 15 articles that directly match on the inclusion criteria as studied in Table 1.
| Author published year | Type | Study population | Outcomes |
|---|---|---|---|
| Keystone et al. 2007 | Clinical trials | 1Kg × 2 + MTX | Subanalysis of open phase, patients with initial response |
| Rubber Roth et al. 2010 | Clinical trials | Active RA | ACR response, EULAR response |
| Ronald et al. 2012 | Pooled observed case analysis | Patients with moderate-to-severe, active RA treated with rituximab | Rituximab remains generally well tolerated over time and multiple courses, with a safety profile consistent with published data and clinical trial experience |
| Seung et al. 2019 | Double blind trial | RA patients | Switching from US-RTX or EU-RTX after two treatment courses had no adverse effect on the efficacy |
| Ga young et al. 2020 | Retrospectively analyzed the medical records | Patients with refractory IIM treated with RTX | RTX could be an effective and relatively safe therapeutic option in patients with refractory IIM |
| Parvathypriya et al. 2021 | Single-centre retrospective analysis | Treating RA patients with RTX in a protocol-based manner | Population were able to achieve moderate to good control of disease activity |
Table 1: Characteristics of the study including in this review analysis.
Mechanism of action of rituximab in RA
Rituximab targets the CD20 molecule, which is expressed neither on the surface of B cells from pre-B-cell through memory B-cell stages but not on stem cells and pro B cells nor on plasma cells/blasts. Rituximab leads to transient but almost complete depletion of B cells in the blood and only partial depletion in the bone marrow and synovial tissue. Response has been shown to correlate with the level of synovial membrane B-cell depletion and early peripheral blood depletion of B cells measured by sensitive assays, possibly useful as a surrogate. It also frequently induces a reduction of immunoglobulin, notably IgM (see supplementary material, available online only, for more detailed discussion) [5]. B-cell repopulation studies following rituximab treatment suggest reconstitution with antigenically inexperienced, transitional B cells derived from an immature population. In some patients, B-cell repopulation leads to a relapse of the disease. However, further investigations to be able to clarify clear patterns predictive of relapse are still needed [9].
Serious infections
Rituximab does not seem to increase the risk of infections in patients with HIV with lymphoma (category IIb). In the oncology literature, rituximab does not markedly add to the risk of infections induced by chemotherapy; this includes opportunistic infections and also herpes zoster infections, although there was one case of disseminated and fatal herpes zoster infection [10]. In the long-term safety analysis of RA trials herpes zoster occurred in 2% of patients (n=49; 0.98 events/100 patient-years) with only one case a serious AE; this rate appears to be similar to the rate seen with TNF inhibitors [11].
Long-term safety of rituximab
A recent pooled analysis of safety data from the rituximab in combination with methotrexate global clinical trial programme were based on 5013 patient-years of rituximab exposure (n=2578 having received at least one course of rituximab). The rate of AE and serious events including infections and serious infections remained stable across several courses [12].
It is concluded that rituximab is effective primarily in seropositive RA. Recent studies have further supported the efficacy of reduced dosage and different regimens, although more work is needed to establish the optimal strategy. Safety data from rheumatology as well as oncology literature highlight the need for hepatitis screening as well as checking pre-treatment immunoglobulin levels to identify patients possibly at greater risk of infection. Data thus far do not indicate the need for routine tuberculosis screening. As with other biological agents, the need for vaccination should be assessed. Safety concerns for very rare events such as PML have emerged. Ongoing evaluations should clarify the remaining open issues and ultimately lead to a more refined application of rituximab therapy.
Citation: Mehmood M, Arshad M (2021) Effectiveness and Safety of Rituximab in Patients with Active Rheumatoid Arthritis. Rheumatology (Sunnyvale). 11.283.
Received: 27-Apr-2021 Accepted: 11-May-2021 Published: 18-May-2021 , DOI: 10.35248/2161-1149.21.11.283
Copyright: © 2021 Mehmood MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.