
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research - (2022)Volume 12, Issue 3
The present study aimed at evaluating the effect of thiacloprid on reproductive hormones and histopathology of testes and ovaries of rabbit Oryctolagus cuniculus. For this purpose, 72 rabbits, both male and female rabbits were distributed in 4 groups having equal sex ratio (1:1), i.e., each group having 9 male and 9 female rabbits. Group A was kept as control group while group B, C and D were treated with 10 mg, 20 mg and 30 mg respectively for 45 days. Specimens were analyzed on 15th, 30th and 45th day of study. A significant decrease (P ≤ 0.05) in serum level of hormones, e.g., testosterone, estrogen and progesterone was recorded during the study. Histopathological studies revealed tumor formation, haemorrhages, tissue damage and degeneration, formation of cyst in ovaries, while contraction of seminiferous tubules, broadening of interstitial space, decreased number of leydig cells and haemorrhage in testicular tissues. It was concluded that thiacloprid is toxic to reproductive organs and cause damage in ovarian and testicular tissues. It is recommended that application of thiacloprid must be limited in order to prevent damage to non-target species.
Pesticides; Neurodegeneration; Neonicotinoid; Estrogen; Progesterone; Testicular cells; Ovarian cyst; Tumor formation; Thiacloprid; Hemorrhage; Specimen; Testosterone
For past few decades, an extensive increase in the usage of insecticide has been observed all around the world. Insecticides are applied to kill the insects and increase the productivity of crops [1]. World Health Organization (WHO), has defined insecticides as “toxic compounds used to control population of pests, i.e., insects, rodents, fungi and undesirable plants and also used for public health concern such as to decrease the insect borne diseases” [2]. There are three main divisions of neonicotinoids on the basis of level of toxicity includes Ncyanoguanidines, nitromethylenes and N-nitroguanidines. Thiacloprid belongs to class N-cyanoguanidines [3]. Neonicotinoids spread into the entire plant body as it shows systemic effect on the pest. They act on post-synaptic nicotinic acetylcholine receptors and block them. Blockage of receptors leads to decrease in impulse transmission [4]. As a result, paralysis and death of the insects occur. These receptors are present in the central nervous system and are very sensitive for their action [5]. These chemicals contest with neurotransmitter and irretrievably bind with acetylcholine receptor. Prolonged action potential causes extreme flow of ions such as Ca+2, Na+ and K+. Over excitation of neuron causes severe paralysis and ultimately leads to death [6].
Mild poisoning of thiacloprid induces headache, vomiting, dizziness, diarrhoea, nausea, stomach pain and drowsiness [7]. Severe poisoning by this chemical includes hypothermia, pneumonitis, metabolic acidosis, hypotension, and ventricular dysrhythmias. Acute toxicity can be induced by inhalation and swallowing [8]. It can affect throat and nose even by smelling and can cause irritation in eye. Damage can be extended to other organs by long term exposure [9]. World Health Organization has categorized it as intermediate poisonous pesticides (class II) [10]. It is known to show carcinogenic effect too. Tumors can be induced in urinary tract, thyroid and ovary in rats [11].
Procedure of action of thiacloprid includes interfering with chemical signal transmission, nerve action potential and muscle paralyses. This chemical is very stable in light and confronts the action of rain [12]. Thiacloprid accumulates in plant tissue and transferred to higher mammals by food [13].
Estimation shows that thiacloprid have long term effect on fresh water organisms even temporarily exposed. Sensitivity of animal is considered as the important factor for evaluating the influence of pesticide on animals. Even 5% thiaclprid is dangerous for several water bodies. Rain and storm runoff is quite responsible for transfer of pesticides to water bodies [14].
Few scientists studied the sensitivity of birds to insecticides. Data showed histopathological changes in morphology of liver, lungs and intestine due to repeated uptake of thiacloprid for 28 days by using G. domesticus [15]. The cerebral hemisphere had exposed changes comprising of insignificant neuronal disintegration with surrounding glial cells, satellitosis and vacuolation. The oral sub-acute toxicity revealed adequate risk in G. domesticus. Stress was provided by 2 ml of glass syringe and repeated oral dose of 10 mg/kg/day was used. Post-mortem was used for systemic findings from every organ of bird. Significant enlarged liver, haemorrhage, necrosis and other physiological changes showed the destructive effect of thiacloprid [16].
Thiacloprid was bought from Faisalabad with commercial name Garner. Mammalian species closely related to Homo sapien should be chosen for histopathological studies. Healthy 72 rabbits Oryctolagus cuniculus weighing about 1.0 kg to 1.5 kg had been nominated as an experimental animal, immunized and acquired before two weeks for acclimatization purpose from disease free store of Gujranwala and divided into four groups namely A, B, C and D. The rabbit (Oryctolagus cuniculus) is the small sized mammal closely linked with “Leporidae” and order “Lagomorpha”. For acclimatization a huge room with partition was used in which rabbits were kept in controlled light condition [17]. Control group A served no dose and is called normal group group B 10 mg/kg for low dose, 20 mg/kg for medium dose and 30 mg/kg for high dose [18]. Doses were orally treated with food. These doses were repeated for 45 days. First trial for haematological test and histopathology were taken at 15 days. Second and third trial is taken after 30 and 45 days respectively. Meanwhile, 6 rabbits were slaughtered on 15th, 30th and 45th day from each group and histopathological examination of gonads i.e., testes and ovaries were conducted [19].
Hau and Schapiro procedure for blood collection from rabbits was used. Total blood volume is 6% of lean body weight. Maximum 2% of body weight blood was collected every one month [20]. For hormone tests (ELISA protocol) were performed to study deviation in the level of sex hormones. Estrogen test kits, progesterone test kits, testosterone test kits, estrogen-HRP-conjugate concentrate progesterone-HRPconjugate concentrate, testosterone-HRP-conjugate concentrate, estrogen IgG coated microtiter Plate (12 × 8 wells=96 wells), progesterone IgG coated microtiter plate (12 × 8 wells=96 wells), testosterone IgG coated microtiter plate (12 × 8 wells=96 wells), TMB ELISA reagent (for 1 step 11 ml), incubator, magnetic stirrer, vials, pipettes (1.0 ml, 200 μl, 100 μl, 50 μl and 25 μl), pipettes tips, plate shaker, microliter plate reader, absorbent paper or paper towel, automates microplate strip washer, vortex mixer, stop solution and distilled water was used to performed ELIZA [21].
For histological examinations of testes and ovaries, microtomy was performed. The method of Hau and Schapiro was used for the slaughtering of rabbits [17,22]. The sex organs that are ovaries and testes were removed from each of the dissected rabbit which is required for histological examination. For cleaning, 0.085 saline solutions were used [23]. Pieces of about 3-4 mm of ovary and testes were cut on sterilized dissecting box. Tissue fixation is an important step in the process of histopathology [24]. Tissues were put in Bruin’s fixative for about 7-8 hours. Protein was thickened by fixative and could not dissolve in alcohol. Series of alcohol used to eliminate the colour of bruins’ fixative. 70% alcohol (1-2 hours), 90% alcohol (1-2 hours) and Absolute alcohol (half hour) [25]. After dehydration, the organs were dipped in clove oil for over the night to provide them a rubbery texture which was required to isolate the section [26]. The next day the organs were removed from clove oil and subjected to Xylene I and Xylene II for 1.5 hours each. Slices of tissues embedded in wax and then trimmed. Trimmed wax block was melted from one side to attach it with wooden chuck with the help of spirit lamp flame [27]. Meanwhile, tissue floating bath was switched on and set at the temperature of 45°C [28]. Fine waxy tissue slice cut from microtome machine and mount it on slide by using Mayer’s albumin egg white [29]. After that staining, a dewaxing and rehydration process was done. Haematoxylin and Eosin stain were used. The desired sections (testes and ovaries) were studied by using the microscope having digital camera and finally micrographs were taken [19,30].
For data analysis, SPSS (Statistical Package for Social Science) version 21 was used. Standard error mean (± S.E.M) and mean were also calculated in addition to other desired parameters. Moreover, one-way ANOVA test used for determining the variation among groups for data analysis and subsequently, post hoc test at 0.05 probability level was also determined to check how many variations were found within these groups.
The body weight of male and female rabbits decreased as a result of thiacloprid administration, especially of medium dosage group of female. Significant effect was detected on the reproductive hormones and reproductive organs of rabbits. An obvious decrease was observed in hormonal production. Urine of treated rabbits was more concentrated as compared to control group. Oxidative stress was also considered as a factor of damage to ovaries and testis. Hormonal test evaluated the significant reduction (P ≤ 0.05) in all male and female reproductive hormones that is estrogen, progesterone and testosterone level (Figures 1-3). Histopathological studies elucidated the reduction in follicular diameter, cyst formation haemorrhage and damage to ovarian tissues was observed in female rabbits (Figure 4). Whereas in male rabbits, increased interstitial space, reduced number of Leydig’s cells, reduced lumen of sertoli cells, tumor formation, tissue damage and haemorrhage showed the signs of reproductive toxicity in treated groups as compared to control group (Figure 5).
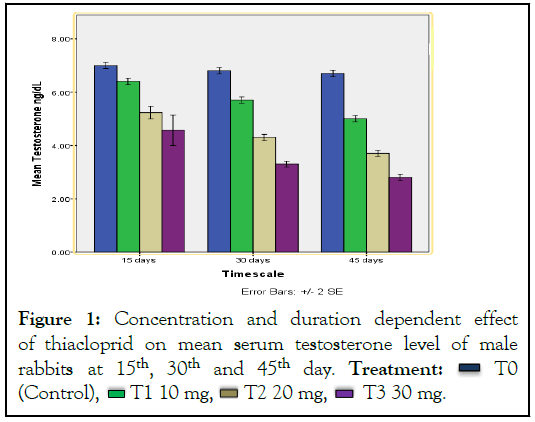
Figure 1: Concentration and duration dependent effect
of thiacloprid on mean serum testosterone level of male
rabbits at 15th, 30th and 45th day. Treatment:  T0
(Control),
T0
(Control),  T1 10 mg,
T1 10 mg,  T2 20 mg,
T2 20 mg,  T3 30 mg.
T3 30 mg.
Figure 2: Concentration and duration dependent effects
of thiacloprid on mean serum estrogen level of female
rabbits at 15th, 30th and 45th day. Treatment:  T0
(Control),
T0
(Control),  T1 10 mg,
T1 10 mg,  T2 20 mg,
T2 20 mg,  T3 30 mg.
T3 30 mg.
Figure 3: Concentration and duration dependent effects
of thiacloprid on mean serum progesterone level of female
rabbit at 15th, 30th and 45th day. Treatment:  T0
(Control),
T0
(Control),  T1 10 mg,
T1 10 mg,  T2 20 mg,
T2 20 mg,  T3 30 mg.
T3 30 mg.
Figure 4: Effect of thiacloprid on ovaries of group C and D at 45th Day. Note: Taken from labomed microscope (FLR L X400).
Figure 5: Effect of thiacloprid on testes of group C and D. Note: Taken from labomed microscope (FLR L X400).
The present study indicates that thiacloprid has dose-dependent adverse effect on testes and ovaries of male and female rabbit respectively. Histopathological study reveals that the tissues seem to be damaged. In addition, it is responsible for many histopathological and hormonal changes in male and female rabbits. Hormonal release is decrease along with histopathological alterations of testes. It also reduces the level of progesterone and estrogen secretion. It causes severe damage to ovaries and produce ovarian tumor. Moreover, it produces multiple effects such as haemorrhage, tissue disruption, and cyst formation in females, epithelial lining disruption in both genders, widening of interstitial spaces, death of leydig cells and narrowing of seminiferous tubules in males. Significant changes (P ≤ 0.05) observe in hormone production level by all thiacloprid doses. In order to prevent damage in non-target organism we should regulate the dosage concentration of thiacloprid in crops. Keeping in view the result of this study and the usage of this chemical should be limited in order to prevent damage in mammals. The application of thiacloprid should be prevented near water bodies to lessen the drastic effects in aquatic organisms.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Islam A (2022) Effect of Thiacloprid on Reproductive Hormones and Histopathology of Reproductive Organs of Rabbit (Oryctolagus cuniculus). J Clin Toxicol. 12:513.
Received: 08-Jun-2022, Manuscript No. JCT-22-17832; Editor assigned: 10-Jun-2022, Pre QC No. JCT-22-17832 (PQ); Reviewed: 24-Jun-2022, QC No. JCT-22-17832; Revised: 01-Jul-2022, Manuscript No. JCT-22-17832 (R); Published: 08-Jul-2022 , DOI: 10.35248/2161-0495.22.12.513
Copyright: © 2022 Islam A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.