Research Article - (2022)Volume 6, Issue 1
In endodontic, the treatment aims to eliminate infection and necrotic tissue of the root canal and fill the threedimensional space of the root canal system to prevent the passage of liquids and microorganisms. In this regard, root canals are commonly filled with gutta-percha points in combination with an endodontic sealer. Hence, a proper root canal sealer is necessary to establish a fluid-tight cover between the dentinal wall of the root canal, the gutta-percha, and the apex. For many decades, it has been maintained that an adequate sealer must not interfere with the healing processes and repair of the apical and periapical tissues.
Though, sealers tent to be extruded into the periodontal tissues. The majority of the research states that all root canal sealers, independently of the type, exhibit toxicity in their freshly mixed state. Then, the toxicity decreases after setting, and the cement becomes relatively inert [1-3]. AH Plus, a resin epoxy-based sealer, is considered the gold standard of endodontic sealers, due to its excellent physicochemical properties. Also, its composition includes radiopaque fillers, such as calcium tungstate and zirconium dioxide [4]. Previous studies showed that materials containing epoxy resin produced cytotoxicity [5,6] and stimulated the inflammatory response [7]. Advances have been made in root canal sealers, particularly with tricalcium silicatebased sealers, also called bioceramic sealers. The preparations are presented as a powder and liquid; the powder is composed of tricalcium silicate and zirconium oxide (opacifier), and the aqueous solution is made of calcium chloride and excipients [8,9]. These modifications provide advantages compared to traditional sealers, improving the physicochemical and biological properties of the sealers. For example, they have better handling properties and prevent tooth discoloration. Moreover, periapical and periodontal tissues, as well as the alveolar bone, have demonstrated favorable responses and tolerance to tricalcium silicate-based sealers. Also, tricalcium silicate-based sealers release hydroxyapatite during the setting reaction, favoring osteoconductivity. Additionally, the sealers have an alkaline pH during the first 24 h of setting, resulting in an antibacterial action [8,10,11]. These properties aid to confine the sealer and the gutta-percha within the root canal space. However, extrusion of the materials often occurs during obturation procedures. The contact between the sealer and the soft and hard apical tissues can cause continuous inflammation of peri-radicular tissues and may result in delayed wound healing. In this regard, periodontal ligament cells represent target cells in endodontic treatments due to their biological response during apical sealing. Also, their interaction with endodontic materials is considered critical for the treatment outcomes. This study aimed to compare the adhesion and proliferation of human periodontal ligament fibroblasts in transverse sections of premolar teeth sealed with two root canal sealers. The root model of the present study aimed to simulate the direct contact between the sealers and periodontal ligament cells, similar to clinical settings. Furthermore, we aimed to provide a more in depth understanding of periodontal tissues' clinical response to endodontic treatments.
Cell culture materials and reagents were from Corning Materials Science Technology and Innovations and Sigma-Aldrich, USA. Root canal sealers, AH-Plus® was from Dentsply sealers (DeTrey, Konstanz, Germany) and BioRoot™ RCS was from Septodont, (Saint Maur DesFosses, France). All tissue samples were obtained from healthy patients following the guidelines established by the Research and Ethics Committee of the School of Dentistry athe UNAM (Number, CIE/1110/2017), and the Mexican legislation for research in human subjects (NOM-012- SSA3-2012) Primary human periodontal ligament fibroblast cell cultures. Human periodontal ligament fibroblast cells (hPDL) were prepared from immature third molars freshly extracted for orthodontics reasons in compliance with maxillofacial surgeryclinic by the explant outgrowth method [12]. Cells were culturedin minimum essential alpha medium (α-MEM) supplemented with 10% fetal bovine serum, 100 UI/mL penicillin, 100 mm/mL streptomycin, and 0.25 mg/mL amphotericin B at 37°C in a 95% air 5% CO2 in atmosphere. α- MEM (MEM supplemented with 10% fetal bovine serum, 100 UI/mL penicillin, 100 mm/mL streptomycin, and 0.25 mg/mL amphotericin B) was used for further experimentation. Processing of material for hPDL cell seeding. The written informed consent was obtained from all the patients or their parents. Intact freshly extracted maxillary and mandibular premolars with single roots were stored at 4°C in saline solution (supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 0.25 mg/mL amphotericin B). The crowns were removed at the cement-enamel junction with a diamond disk under cold water. A size 10 Kfile Mailefer was used to ensure apical patency and measure the working length. The apical patency was ensured by visualizing the 10 K-file tips at the root apex. When this was not possible, roots were discarded. Kfiles of increasing diameter were used to measure the apical diameter at the working length before instrumentation. Teeth with an open apex were excluded. Finally, 30 teeth were selected and used. The root canals were prepared with rotatory system Protaper Next (Dentsply Maillefer, Switzerland) with the elements motor (SybronEndo® ).The instrumental sequence was as follows: File X1 (size 17, .04 taper), X2 (size 25, .06 taper) and X3 (size 30, .07 taper); the irrigation was consistent with sodium hypochlorite at 2.5%. Cross-sections of the roots of 2 mm were made, only sections from the central area of the root and with similar dimensions were selected. A total of 60 cross-sections were used per group. The 30 teeth were divided randomly in 2 groups using continuous-wave condensation technique (AH-Plus® + guttapercha {n=15}) or single cone technique (BioRoot™ RCS+ guttapercha {n=15}). After sterilization under ultraviolet light for 15 min, the teeth were then stored for 1 day in an incubator at 37°C to allow the setting of the root filling materials. BioRoot™ RCS and AH-Plus® (were prepared according to manufacturers’ instructions (Figure 1).
Figure 1: The root model experimental procedure.
Dental students with insufficient clinical experience are more likely to be exposed to infectious diseases. Thus, dental students and practitioners need to be cautious and show high compliance with the preventive strategies to minimize the spread of COVID-19. Previous studies have shown that dental practitioners, including students, show inadequate knowledge and inappropriate attitudes toward infection control measures. Therefore, this study aimed to investigate the awareness and attitude of dental students for the appropriate use of face masks during the COVID-19 pandemic in Saudi Arabia.
It were removed at the cement-enamel junction, B) 30 teeth were selected, C) Cross-sections of the roots were made of 2 mm, D) The experimental groups were obturated with BioRoot RCS or AH-Plus, E) Human Periodontal Ligament cells were seeded onto the root slides for biological evaluation. Cellular adhesion assays. The hPDL cells were cultured in minimum essential alpha medium (α-MEM) supplemented with 10% fetal bovine serum, 100 UI/mL Penicillin, 100 mg/mL of Streptomycin, and 0.25 mg/mL of Amphotericin B at 37°C in a 95% air 5% CO2 atmosphere. Human PDL cells were seeded at 1 × 104 cells/mL onto the root slice placed in 48-well culture plates and allowed to adhere under standard cell culture conditions for 4 and 24 h. After the prescribed time, the root slice was rinsed three times employing PBS to remove no adherent cells. The evaluation of cell attachment was performed according to a crystal violet assay. Briefly, adherent cells were fixed with 4% paraformaldehyde and incubated with 0.1% crystal violet solution for 15 min. Then, the dye was extracted with 0.1% of Sodium Dodecyl Sulfate(SDS) and optical absorption was quantified by spectrophotometer at 545nm with a plate reader (ChroMate, Awareness Technology) . Cell proliferation assay The cell viability of hPDL cells plated at a concentration of 1 × 104 cells/mL onto the roots was checked by the WST-1 assay for 3, 7, 9, 14, and 21 days of culture. This assay is based on the ability of the mitochondrial succinatetetrazolium reductase enzyme, secrete by living cells, to reduce a WST-1 salt (4-(3-(4-iodophenyl)-2-(4-nitrophenyl)-2H5- tetrazolium)-1,3-benzene desulphonated) to produce a watersoluble formazan dye product. Hence, the formazan product concentration is directly proportional to the number of metabolically active cells [14]. Human PDL cells seeded onto the roots slice at the prescribed times were washed with PBS and incubated with 400 μL fresh culture medium containing 40 μL of the cell proliferation reagent WST-1 for 4 h at 37°C. Then, 200 μL of the supernatant was removed, and the absorbance was quantified by spectrophotometer at 450 nm with an plate reader (Chromate, Awareness Technology). During the experimental time, the medium was changed to fresh supplemented medium every 3 days. The experiment was done in triplicate, with three repetitions for each condition every time.
Cell morphology both materials were incubated with α-MEM for 24 h and then evaluated with fluorescence microscopy. Briefly, before seeding onto the root slices, the hPDL cells were incubated with Cell-Tracker™ Green CMFDA (5- chloromethyl fluorescein diacetate) in phenol red-free medium at 37°C for 30 min. Subsequently, the cell culture was washed with PBS and incubated for 1 h in complete medium. After recovery, hPDL cells were trypsinized and counted to the desired cell concentration (1 × 104 cells/mL) and incubated for 24 h onto the root slices. The morphology of the cells was subsequently evaluated, particularly their spreading pattern and cell-material.
Statistical analysis all quantitative data were expressed as mean ± standard error. Data were analyzed by Student’s t-test to determine the differences among the groups. Statistical significance was considered at p<0.05.
Adhesion assay the response of cell adhesion of hPDL cellsseeded onto tooth slice was analyzed by crystal violet according to the defined time intervals (Figure 2). The results showed that more cells adhered to the root slices treated with BioRoot™ RCS than on the slices treated with AH-Plus®. However, both treated root slices showed higher adhesion when compared with control after 4 and 24 h of cell culture (p>0.05). The result suggests that the filling material are contributes significantly to the attachment of hPDL cells, and the fluorescence staining supported the results regarding cell adhesion.
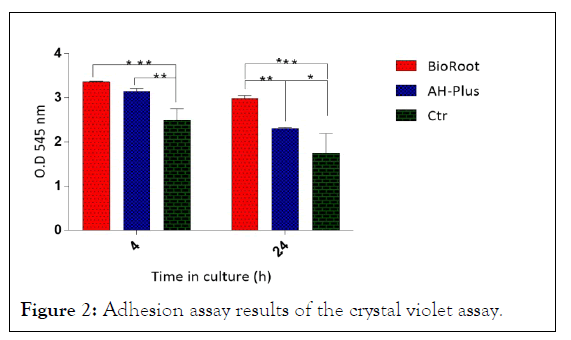
Figure 2: Adhesion assay results of the crystal violet assay.
Proliferation assays. The cell viability of hPDL cells cultured onto tooth slice treated with BioRoot™ RCS and AH-Plus® after seeding for 3, 7, 14, and 21 days was characterized by the WST-1 assay (Figure 3). The results showed that after 14 days of culture, the cell viability of hPDL cells cultured onto tooth slice treated with BioRoot™ RCS has a significant difference over the slice treated with AH-Plus® (p<0.05). While at the 3, 7, and 21 days after cultivation, the cell proliferation is maintained, with slightly higher and constant growth for the BioRoot™ RCS treated root slides. These results showed that the filling material did not induce any cytotoxicity effects on the hPDL cells, and the material improved the viability and the proliferation of cells. Also, the results demonstrated that the experimental groups showed an increase in cell growth compared to the controls.
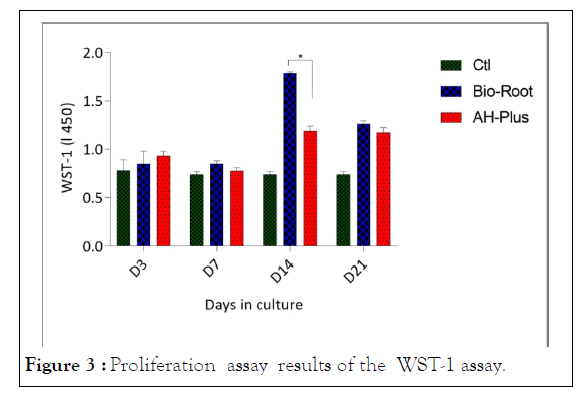
Figure 3: Proliferation assay results of the WST-1 assay.
It proliferation after 3, 7, 14, and 21 days in culture withBio-Roo or AH-Plus. Fluorescence. To determine whether cells could grow, attach, and interact with the material, fluorescence analysis was performed to observe the morphology of cultured hPDL cells onto the material. The fluorescence analysis showed that hPDL cells adhered and grew on the surface of the root model. The hPDL cells adhered to the root slices filled with BioRoot™ RCS, uniformly covered all the surface or the slice, and showed positive staining represented by a green apple color, and this was interpreted as adequate viability of the cells. The cells maintained a large, flat, elongated (spindle-shaped) morphology, as shown by the fluorescence images, as showed in Figure 4.
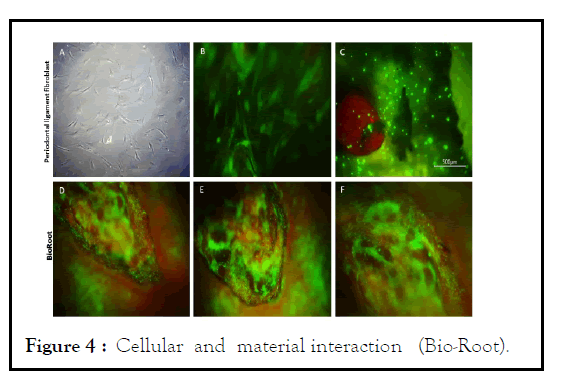
Figure 4: Cellular and material interaction (Bio-Root).
Microscopic evaluation of Human Periodontal Ligament cells (hPDL) in the in vitro root model in the presence or absence of BioRoot. Representative images of the morphology of hPDL cells seeded onto a tissue culture plate observed under an inverted microscope (A), magnification PL 4X. Incubation of hPDL cells with Cell-Tracker™ Green CMFDA after 30 minutes (B), magnification 20X. Fluorescence image of the cell-material interactions in the experiment corresponding to BioRoot (C, D, E, F), magnification 4X. (C) hPDL cells onto control slice without any presence of the material, scale bar of 500 micrometers. (D, E, and F) hPDL interaction over the surface of BioRoot that shows a better biocompatibility whit the material, magnification 10X. Moreover, the hPDL cells seeded onto the root slices filling with AH-Plus® (Figure 5) also showed affinity to the experimental surfaces, with isolated cells or with cells in small disperse groups over the surface, presenting few spreading cells that exhibited a flat shape as shown in the fluorescence microphotographs. Also, the cell-material interaction analysis of the spreading and morphology of hPDL cells showed that the filling material did not induce any visible changes in the cellular morphology, such as hydropic degeneration, granular to vacuolate cytoplasm or cell membrane rupture. Together, the results suggest that the filling material is not toxic, and indicate that the filling materials favor the spread of hPDL cells, and support cell adhesion. The results from the proliferation and adhesion assays demonstrated that hPDL cells adhered to a higher degree on the root slices obturated with BioRoot™ RCS. The results also indicated that the BioRoot™ RCS root slides were more favorable for cell adhesion and proliferation than the AH-Plus®.
Figure 5: Cellular and material ineaction (AH-Plus) .
Microscopic evaluation of Human periodontal ligament cells (hPDL) in the in vitro root model in the presence or absence of AH-Plus. Representative images of the morphology of hPDL cells seeded onto a tissue culture plate observed under an inverted microscope (A), magnification PL 4X. Incubation of hPDL cells with Cell-Tracker™ Green CMFDA after 30 minutes (B), magnification 20X. Microscopic evaluation of Human periodontal ligament cells (hPDL) in the in vitro root model, corresponding to AH-Plus (C, D, E, F), magnification 4X. The hPDL cells onto control slice without any presence of the material (C), magnification 4X, scale bar of 500 micrometers. The hPDL interaction over the surface of AH-Plus showed adhesion to the material, a uniform spreading pattern, and morphology similar to the control, magnification 4X, interpreter as a favorable biocompatibility whit the material.
This study aimed to compare the adhesion and proliferation of human periodontal ligament fibroblasts cells (hPDL) in transverse sections of slice teeth sealed with different root canal sealers. Several studies have investigated the degree of cytotoxicity of root canal sealers on periodontal ligament cells is of the investigated. However, few models evaluate cell cytotoxicity in similar conditions to those of sealing in human teeth, as was performed in this study. The dental organs were instrumented with the protocol used in the clinic and filled with the continuous wave condensation technique (AH-Plus® + guttapercha) or single cone technique (BioRoot™ RCS+guttapercha), similar to settings in the oral cavity, thus managing to reproduce closely the contact that the sealing material has in the oral cavity.
However, few models evaluate cell cytotoxicity in similar conditions to those of sealing in human teeth, as was performed in this study. Besides, when a significant amount of the material is extruded, it can induce cytotoxic damage to the periodontal tissues. In the present study, a biological evaluation was measured at different culture times to observe the biocompatibility.
Despite their solubility, the di- and tricalcium silicate cement at the beginning of the setting process release ions of OH- y Ca2+, producing calcium hydroxide formation, which contributes to their antimicrobial efficacy. Also, hydroxyapatite is formed on the surface of the materials, promoting theirbioactivity. In this study, it was observed that this effect influenced the values obtained in cell adhesion assays, which were higher in BioRoot™ RCS than in AH-Plus®. However, when cell proliferation was evaluated, it was decreased in BioRoot™ RCS root slides compared to AH-Plus® root slides, attributed to the solubility of the BioRoot™ RCS. Through the course of the experiment, we could observe that after soaking the root slices in the culture medium, a uniform sediment and granular material deposits were at the bottom of the culture plates that preserved the samples sealed with BioRoot™ RCS. The sediments might represent solubility and loss of the material, characteristics previously reported for BioRoot™ RCS. However, in the present study, the BioRoot™ RCS sealer had better longterm stability, which in turn maintained the cell growth onto the material. In contrast, the cell proliferation in the BioRoot™ RCS root slides was better than in cells seeded onto AH-Plus® root slides. Moreover, cell proliferation was higher in the experimental groups than the control.
The majority of cytotoxicity studies performed with sealers have been carried out directly on the tabled-shaped material, which generally led to more significant limitations in not imitating its clinical use. In the present study, the manufacturer’s protocol was followed, and the sealers were used following parameters apply in clinical practice, which allows us to obtain results much closer to clinical use.
In endodontic therapy, it is essential to make a seal as hermetic as possible, since a three-dimensional seal is essential to prevent re-infection and re-contamination; i.e., the prevention of the passage of microorganisms and toxins to the periapical tissues. It has been reported that all sealing techniques by thermoplastification will reproduce the defects and irregularities of the root canal. Also, it has been argued that a continuous wave seal grants a filling with fewer spaces, as opposed to the with the BioRoot™ RCS have been perceived to be less effective, due to the solubility of the material, which could compromise the quality of the root canal treatment and reduce the hermetic sealing of the root canal. However, the present study showed that BioRoot™ RCS favored proliferation, and worked as a platform to improve cell biocompatibility (being not toxic to cells), which eventually provided support to colonization and cell growth of periodontal fibroblast cells.
The filling material gives to the tooth slice a property that is important on endodontic treatment because the success of the root canal treatment depends on the manipulation and the filling material; i.e., a specific microenvironment by filling material that improves cell biological response allowing the promotion of fibroblast activity . This study showed that both types of sealers allowed the growth of hPDL cells in vitro; thus, in the case of extrusion of sealant cement, periodontal tissues are expected to exhibit biotolerability. In addition, BioRoot™ RCS and AH-Plus® sealants exhibited excellent cytocompatibility in terms of cell adhesion at 24, with a significant statistical difference and the highest proliferation rate found in the BioRoot™ RCS root slides, followed by the AHPlus ® sealant cement. Also, significant statistical differences in terms of cell proliferation were observed after 14 and 21 days of culture for the AH-Plus® root slides. Previously published research demonstrated that BioRoot™ RCS presents a higher solubility at the beginning, then, the solubility decreased with time. As a result, the leaching of these toxic substances decreased, consistent with the results observed in the present study.
The low cytotoxicity of endodontic sealant cement is one of the basic conditions for a successful endodontic treatment and healing of the periodontium. Therefore, considerations for choosing suitable root canal sealant cement include both its physical and biotolerability properties. The toxicity that endodontic sealant cement can cause to periapical tissues is essential. However, endodontists must evaluate the advantages and disadvantages of sealant extrusion, as well as the sealing technique. Although no statistically significant difference has been found in the percentage of filling material volume, it has been reported that currently available sealers shrink slightly upon setting, which also should be taken into consideration.
The results presented here confirm that there are differences between BioRoot™ RCS and AH-Plus®, suggesting that both materials possess excellent biocompatible properties, allowing cell growth after 21 days of culture. The best results were obtained for the BioRoot™ RCS root slides, after 24h, and after 21 days of culture. Interestingly, AH-Plus® demonstrated the best results after 14 and 21 days, which could be related to the cement’s solubility and the decreased of toxic substance release by the sealant.
Also, in the evaluation of cell morphology, hPDL cells seeded onto BioRoot™ RCS showed a superior spreading pattern compared to those in contact with AH-Plus® root slides. However, in both experimental groups, hPDL were able to adhere to the root surface, and to the obturation material.
Further in vitro and in vivo studies are required to confirm the suitability of calcium silicate-based and resin epoxy-based endodontic sealers for clinical application. An in vitro scratch wound-healing model would be used to determine their effects in cell migration and to assess cell morphology and attachment analyses by scanning electron microscopy . Moreover, this type of work performed in vitro showed that BioRoot™ RCS is associated with better biological response than AH-Plus®, and may well be regarded as an alternative for endodontic treatments, improving its clinical relevance.
These in vitro findings revealed that BioRoot™ RCS and AHPlus® have bioactive properties that modulate adhesion and proliferation of hPDL cells. The hPDL cells contribute to apical sealing. Therefore, their response contributes to a better understanding of the periodontal tissues' regeneration. Also, the similar settings to a clinical scenario, to study the mechanisms of model used in the present study provides a human model, with the materials used in the clinic. It demonstrates that BioRoot™RCS was more effective in short-term use and is a promising material that could facilitate root canal treatment for the successof endodontic applications. However, before their clinical employment, studies regarding their sealing properties also need to be considered.
[Crossref ] [Google Scholar]
[Crossref ] [Google Scholar]
[Crossref ] [Google Scholar]
Citation: Vazquez FC (2022) Effect of Root Canal Sealers on Adhesion and Proliferation of Human Periodontal Ligament Fibroblast. J Odontol. 6:611.
Received: 03-Jan-2022, Manuscript No. JOY-22-45623; Editor assigned: 05-Jan-2022, Pre QC No. JOY-22-45623 (PQ); Reviewed: 19-Jan-2022, QC No. JOY-22-45623; Revised: 24-Jan-2022, Manuscript No. JOY-22-45623 (R); Published: 31-Jan-2022 , DOI: 10.35248/JOY-22.6.611
Copyright: © 2022 Vazquez FC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.