Anatomy & Physiology: Current Research
Open Access
ISSN: 2161-0940
ISSN: 2161-0940
Research Article - (2019)Volume 9, Issue 2
Introduction: Effects of juvenile diabetes on the central nervous system are less well documented. Effective management of T1DM is another major challenge as insulin is the only available effective treatment. Hence, easily available, affordable, non-invasive therapies like enriched environment are the need of time.
Objectives: The present study aimed to determine the effect of an enriched environment on cognitive and behavioral changes in juvenile diabetic rats.
Methods: Diabetes was induced in 25 days old, albino Wistar rats, by streptozotocin (STZ). Animals were reared in an enriched environment and treated with herbal formulae with Salacia R and Clitoria T. Then, rats were tested in the elevated passive maze, passive avoidance box and Morris water maze (n=6). Values are expressed as means ± SE and differences assessed by using one way ANOVA test followed by Bonferroni multiple comparison test.
Results: Statistically significant (p<0.05) difference was observed between the enriched environment and diabetic group of rats. Additionally, effects are comparable to conventional insulin therapy, in the animals which received combined therapy (SR+CT+EE) (p<0.01).
Conclusion: Authors have shown that diabetic rats reared in enriched housing conditions, showed improved cognitive measures compared to diabetics. The combined treatment is found to be more beneficial in restoring normoglycemic levels and preventing behavioral and cognitive deficits.
Latest figures from the International Diabetes Federation-Diabetes Atlas showed that presently 387 million people are living with diabetes. It is expected to rise up to 592.8 million by the year 2035 [1]. Diabetes mellitus is a clinical syndrome, characterized by hyperglycemia due to an absolute or relative deficiency of insulin or non-responsiveness of tissues to insulin. Diabetes causes substantial morbidity and disability, even contributing to the mortality, by causing renal failure and cerebrovascular diseases [2]. Long-standing concern about the deleterious effects of diabetes on CNS has enhanced with the increasing incidence of type 1 diabetes mellitus (T1DM) in children [3]. Many researchers have shown the relationship between neuropsychological changes and T1DM of early onset [4-6]. Diabetes-induced behavioral and cognitive changes to appear to be associated with several factors. However, these changes are less studied and not well documented to date. Hence there is a need for resolving these issues of diabetic encephalopathy in the younger population.
Donald Hebb postulated the concept of the influence of the Enriched Environment (EE) on activity-dependent synaptic plasticity. Since then, a notable number of studies have reported the structural and chemical modifications in the brain following EE [7,8]. The cerebral cortex of the rats showed increased number and size of the synapses, enhanced spine density of neurons as well as electrophysiological maturation of dentate granule cells following the exposure to an EE [9,10]. The exposure to a variety of environmental stimuli for a short period of 10 days was able to enhance proliferation, survival and dendritic arborization of newly born pyramidal CA1 neurons. EE was also reported to increase the vascularity of the dentate gyrus in diabetic animals rescuing the diabetic brain from neurodegenerative progression [11]. Authors have carried out studies to evaluate the efficacy of Salacia reticulata Wight (SR), an anti-diabetic plant [12] and Clitoria ternatea Linn (CT); a nootropic herb [13] in streptozotocin (STZ) induced young diabetic rat pups.
The present study aimed at the evaluation of the efficacy of rearing the animals in an enriched environment (EE) on various neurophysiological (cognitive) and neuropsychological (behavioral) changes in STZ induced young diabetic rats having severe hyperglycemia (FBS ≥ 200 mg/dl). In addition, studies were carried out to determine the effect of combined alcoholic extracts of roots of SR+CT and rearing in EE. Although few studies have investigated the effects of combined herbal therapy in diabetes, seldom any reports are available on the effects of the combination of herbal therapy with an enriched environment in diabetic encephalopathy. No reports were available about the efficacy of SR+CT+EE on diabetic rats. Hence, the present study is unique in evaluating the effects of combined treatment SR+CT+EE on behavioral and cognitive changes in young diabetic rats. Elevated Plus Maze (EPM) Passive avoidance (PA) test and Morris water maze (MWM) tests were used for assessing cognitive and behavioral functions.
Experimental animals
Highly inbred, Wistar strain albino male and female rats weighing about 45 g-55 g were randomly selected and assigned to different groups for the study. The central animal house at J.N. Medical College, Belgaum provided the animals and experiments were carried out after obtaining approval of the Institutional Animal Ethical Committee (627/02/a/CPCSEA dated 17/7/2008). The animals were maintained under 12:12 hours dark: light cycle and fed with standard rat pellets and water ad libitum. The room temperature was maintained at 25 ± 3°C, with relative humidity (55 ± 5%). Animals were handled in a human manner and the minimum required a number of animals used to generate significant data.
In the present study, Streptozotocin (STZ) has been used for the induction of diabetes in young rats [14]. 25 days old rats (approximately equal to 2-2.5-year-old children) were used to determine behavioral and cognitive changes in T1DM of early onset. Animals were induced with diabetes by a single dose of STZ injection (50 mg/kg BW, intra-peritoneal) [15]. The animals in diabetic groups were kept on overnight fasting and received STZ, prepared freshly in 0.1 M cold citrate buffer (pH 4.5). After four days, the blood sample was collected the tail vein of rats to estimate Fasting Blood Sugar (FBS). FBS was estimated with the help of a standard glucometer (Optimum, Germany) after calibrating it with every new set of glucose strips. The day of confirmation of diabetes was referred to as day one of the diabetic state for further procedures in the present study. Animals with FBS levels between 200 mg% and 400 mg% were only included in the study [16]. Body weight of the experimental animals was measured with the help of a standard electronic weighing machine with a resolution of 10 mg. Thereafter, body weight as well as FBS was regularly measured every 15 days during the study period to ascertain the diabetic state of the animals.
Animals were divided in to totally six groups; normal control (NC), diabetic control (DC), treated with insulin (2-6 U/day) (IN), reared in enriched environment (6 hrs./day) (EE), treated with SR and CT combined therapy (each-100 mg/kg.BW) (SR+CT) and treated with SRCT combined therapy and reared in enriched environment (SR+CT+EE). Each group consisted of 6 animals, which were age and sex matched. Each therapy was started from day one of confirmation of diabetic state, the day on which animals were one month old. The diabetic and normal control groups received equal volumes of distilled water (vehicle). Root extracts of Salacia reticulate [17] and Clitoria ternatea [16] were administered orally, once daily for 30 days during the period of treatment.
Enriched housing conditions
Enriched housing was provided as one of the therapies in a specially designed metal cage. The walls of the cage (100 × 60 × 40 cm) were made up of wire mesh to allow enough light and paddy husk bedding was laid at the bottom. The cage was equipped with various exploratory materials like ladders, metal platforms, balls, toys and PVC tunnels of different shape and size. The animals were kept in the cage daily for 6 hours from 10.00 am to 4.00 pm and later replaced to their home cage. During this period, rats were exposed to novelty stimulation by changing the exploratory objects every day; food and water was provided ad libitum [18].
Elevated plus maze
Elevated plus maze (EPM) is a widely accepted test in the study of anxiety and also, sensitive enough to detect deficits in spatial learning and memory in rats [19]. Valid results can be obtained in a short, five-min testing period. EPM consists of two open and two enclosed arms (50 × 10 × 30 cm), attached at straight angles to a central platform (5 × 5 cm). The apparatus is kept in a brightly lit room and elevated to a height of 50 cm above the floor.
a) Anxiety protocol: The rats were placed on the central platform, heading towards an open arm (OA). The number of entries into the open and enclosed arms (EA) were noted and time spent in the open arms was recorded over five min. The decreased time spent and a number of entries into the OA indicates an anxiogenic effect. The frequency of entries into enclosed EA indicates locomotors activity. An arm entry and exit was defined as all four paws entering and two paws leaving the arm respectively. During this period, the animals were simultaneously observed for various ethological parameters such as rearing, grooming, and frequency of excretion.
b) Learning protocol: A midline drawn on the EA divides it into two equal parts. The rats were placed at the end of an OA and allowed to explore for 90 seconds. The transfer latency, time for the rat to cross a line halfway along one of the EA was measured on day one and two. The rat had to have all its four paws on the other side of the midline within 90 sec. If it didn’t cross within 90 sec then rat has to be placed manually and transfer latency was recorded as 90 sec. After crossing the line, the rat was allowed to explore freely for 30 sec. learning was defined as reduced transfer latency on day two compared to day one. Normal rats usually take less time to cross the line halfway on the closed arm compared to the time taken on day one [20,21].
Morris water maze
The Morris Water Maze (MWM) is another well-established and widely used spatial memory test for rodents. Several variants of each of these tasks may be used in order to obtain abundant behavioral indexes of contextual/spatial habituation, cue-driven navigation, operant-like navigation responses learning, and/or decisionmaking. The parameters such as reduced transfer latency and more time spent in the target quadrant are suggestive of improvement in the memory [22-25]. The water maze consists of a circular pool (1.5 m diameter and 60 cm in height,), filled with 30 cm. deep water (26°C ± 1°C) in which a 5 cm2 escape platform was hidden 1 cm below the surface. Water can be made opaque by adding milk or nontoxic color. The MWM was divided into four equal imaginary quadrants. The escape platform was kept in the center of one of the quadrants and the experimental room contained sufficient relevant visual cues.
The rats were trained for three consecutive days. Sessions were of three trials with five min. of inter-trial interval every day. In each trial, the rats were to swim and find the escape platform within 120 sec. In case the rats failed to find the platform within the time limit, then they were hand guided onto the platform and allowed to rest there for 30 sec. The farthest starting location from the platform was used in each trial. The transfer/escape latency is the time taken by each rat to reach the hidden platform. The probe trial was conducted on the fourth day, during which the platform was removed from the maze and the rats were allowed to swim freely. The increase in time spent in the target quadrant which had the platform in the previous trials indicates improvement in the spatial memory.
Statistical analysis
The results of the study are expressed as means and standard errors (mean ± SE). Differences were considered significant at p ≤ 0.05 with a confidence interval (CI) of 95%. Data analysis was done by using SPSS trial version 12. The differences among the therapeutic groups were assessed by using a one-way analysis of Variance (ANOVA) test followed by Bonferroni multiple comparison test. Behavioral parameters in the EPM and PA tests were assessed by the Kruskal-Wallis non-parametric test.
Fasting blood sugar
A statistically significant difference was observed in between the six groups (p<0.001) on post-natal day 30, 45 and 60. The intragroup analysis showed a significant increase in FBS values on postnatal day 30 (p<0.001) and 45 (p<0.001) in DC group and other treated group of animals compared to their normal counterparts. Significant decrease in FBS values was observed on post-natal day 45 (p<0.001) and 60 (p<0.001) in the treated group of animals compared to their respective age-matched diabetic controls. Animals reared in an enriched environment showed significantly increased FBS values compared to that of other animals treated with different therapies (p<0.001) (Table 1).
| Group | Fasting blood sugar (mg/dl) | ||
|---|---|---|---|
| 30th postnatal day | 45th postnatal day | 60th postnatal day | |
| NC | 86.8 ± 3.37 | 92.3 ± 4.65 | 89 ± 3.95 |
| DC | 269.1 ± 20.41* | 276.6 ± 18.99* | 319.33 ± 38.51* |
| SR+CT | 248.33 ± 46.42* | 219.17 ± 46.68*# | 113.67 ± 22.17#$ |
| EE | 241 ± 20.34* | 213.33 ± 42.32*# | 227.67 ± 32.14*# |
| SR+CT+EE | 246.33 ± 37.5* | 164.5 ± 31.38*# | 110.33 ± 15.04#$ |
| IN | 254.17 ± 47.92* | 200.33 ± 29.99*# | 140.83 ± 33.91#$ |
| ‘p’ value | <0.001 | <0.001 | <0.001 |
Table 1: Effects of different treatments on fasting blood sugar levels (n=6).
Performance in an elevated plus maze test
a) Anxiety protocol: A statistically significant difference was observed in the time spent in OA (p<0.001) between six groups. The intragroup analysis showed that animals in the DC group spent significantly lesser time in OA compared to their age-matched controls (Figure 1). A significant increase was observed in the time spent in OA by animals in SR+CT and SR+CT+EE groups compared with their age-matched diabetic controls (p<0.001).
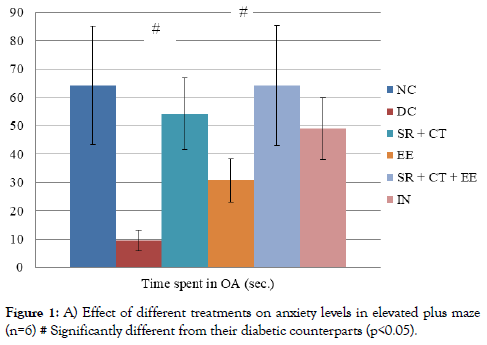
Figure 1. A) Effect of different treatments on anxiety levels in elevated plus maze (n=6) # Significantly different from their diabetic counterparts (p<0.05).
b) Learning protocol: A statistically significant difference was observed between the groups (p<0.001) on day one and two. The intragroup analysis showed a significant increase in transfer latencies among the DC group compared with normal controls on both day one and two. Reduced transfer latencies were observed in animals treated with SR+CT, IN and SR+CT+EE, compared with their age-matched diabetic controls (p<0.001) on day 1 in the learning paradigm. Significantly decreased transfer latencies were observed during memory retention trials, on day 2 in animals treated with different therapies compared to diabetic controls. In addition, transfer latencies were significantly decreased among animals in SR+CT, IN and SR+CT+EE groups compared with their age-matched animals reared in EE. However, EE groups compared to their normal controls, which indicated no improvement with EE (Table 2).
| Group | Transfer latency (in sec.) | |
|---|---|---|
| Day 1 | Day 2 | |
| NC | 46.6 ± 10.07 | 14.1 ± 2.28 |
| DC | 87.8 ± 1.51* | 72.83 ± 14 * |
| SR+CT | 56.8 ± 21# | 13.8 ± 7#$ |
| EE | 71.2 ± 14.6 | 42 ± 12*# |
| SR+CT+EE | 47.3 ± 18# | 17.4 ± 5.9#$ |
| IN | 57.5 ± 25# | 16.8 ± 6.8#$ |
| ‘p’ value | <0.001 | <0.001 |
Table 2: Effects of different treatments on learning and memory in elevated plus maze (n=6).
Performance in Morris water maze
Between groups, analysis showed a significant difference (p<0.001) in the time spent in the target quadrant during the probe test. The intragroup analysis showed that diabetic controls spent significantly lesser time in the target quadrant during the probe test compared to that of a normal and treated group of animals. The statistically significant decrease was observed in diabetic (p<0.001) as well as other therapeutic groups (p=0.001) compared with normal controls. Animals in SR+CT+EE group significantly spent more time (p=0.029) in the target quadrant compared to diabetic controls, suggesting the improved memory retention. However, all other treatment groups did not differ significantly from the DC group, indicating no much improvement in memory after treatment (Figure 2).
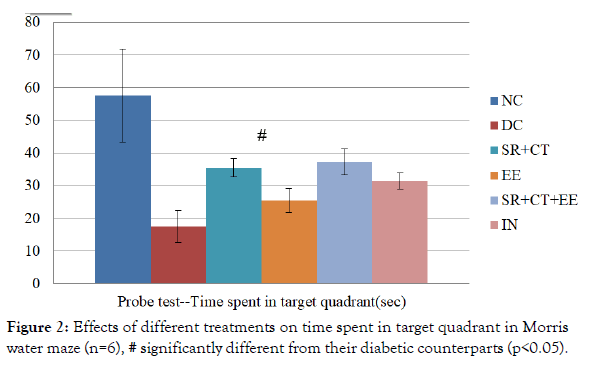
Figure 2. Effects of different treatments on time spent in target quadrant in Morris water maze (n=6), # significantly different from their diabetic counterparts (p<0.05).
Diabetic encephalopathy is one of the thrust areas in neuroscience research. Developing brain of the young is much more vulnerable to the deleterious effects of T1DM of early onset. However, seldom any studies report the role of an enriched environment in diabetic encephalopathy. Authors earlier reported increased anxiety levels and decreased cognition among animals with longer duration of diabetes (20 days) compared with lesser duration (10 days) and normal controls [26,27] In the present study, an effort was made to analyze the efficacy of novel alternative therapies in preventing diabetes-induced cognitive and behavioral changes in young animals. Results of the present study have demonstrated that diabetic animals reared in an enriched environment (EE) showed significant improvement in the learning and memory paradigms compared to diabetic controls. Many researchers have supported the role of an enriched environment in the cognitive enhancement of the younger population in particular. Anatomical and pathophysiological changes in hippocampus were reported along with cognitive deficits. Exposure to EE has been shown to induce neuronal plasticity markedly in both intact and injured adult CNS. It includes up-regulation of multiple neurotrophic factors, resulting in improved spatial learning and memory function. It is reported that enriched animals showed increased cAMP Response Element Binding Protein (CREB) in the hippocampus [28], NGF mRNA levels in visual cortex and hippocampus [18], Nerve Growth Factor (NGF), Brain-Derived Neurotropic Factor (BDNF) and Neurotrophin (NT-3) levels in hippocampus [29,30].
In addition, the present study evaluated the efficacy of alcoholic extracts of roots of Salacia reticulata W. (SR) and Clitoria ternatea L. (CT). The results have demonstrated the efficacy of SR as potent antidiabetic and CT as an effective nootropic agent. The combined extracts have improved the performance of diabetic animals in EPM, PA and MWM tests, which was evident by improved transfer latencies and other parameters. The antidiabetic properties of SR could be attributed to its active fractions such as salacinol and kotalanol, potent inhibitors of α-glucosidases, such as maltase, sucrase, and isomaltase. These actions are analogous with acarbose and miglitol which reversibly inhibit pancreatic α-amylase and intestinal α-glucosidase enzymes. This action results in the lowering of postprandial hyperglycemia due to delayed glucose absorption into the blood. Extracts of Phylanthu semolina, Cassia auriculata, Pterocarpus marsupium were found to have both in vitro and in vivo alpha-glucosidase inhibitory actions [31]. Extracts of Rosa damascene, Punica granatum, Ramulus mori, Myrcia multiflora, and Teco mastans have demonstrated a potent inhibitory effect on alpha-glucosidase activity [32].
The animals treated with alcoholic extracts of CT showed improved learning and memory which could be attributed to increased acetylcholine (ACh) content in the whole brain, decreased acetylcholine esterase (AChE) activity in cortex, hippocampus, medulla and midbrain and increased dendritic arborization of neurons in the amygdala. In vitro studies reported the neurogenic effect of CT on neural stem cells in the anterior subventricular zone, similar to neurotrophic factors like Survivin, Neuregulin 1, FGF-2 and BDNF [33]. The animals treated with CT extracts showed an increased number of entries and spent more time in the open arm of EPM and these anxiolytic effects could be attributed to reducing serotonin levels. Similar nootropic and anxiolytic effect were reported on administration of extracts of Bacopa monnieri [34], Andrographis paniculata [35], Hypericum perforatum, Teucrium polium, Glycyrrhiza glabra [36] and Curcumin [4].
The present study evaluated the efficacy of combined treatment with Salacia reticulata W. and Clitoria ternatea L. along with rearing the animals in an enriched the environment. The animals in SR+CT+EE group showed significant improvement in cognitive and behavioral measures. The improvement may be brought about by synergistic effects due to the combination of herbal extracts and rearing in an enriched environment. The normoglycemic and hypolipidaemic effect of SR may have prevented the hyperglycemia and dyslipidemia induced neuronal damage in the brain [37]. CT may have protected the structural and functional integrity of neurons and synapses [16]. EE is known to enhance synaptic plasticity and increase NGF and BDNF levels in the hippocampus [8]. The animals treated with SR+CT extracts and reared in the enriched environment may have received all these additional benefits. Hence, the results of the present study showed that the animals in SR+CT+EE group showed significant improvement in cognitive and behavioral measures compared to animals treated with insulin, a conventional treatment for T1DM. The animals treated with insulin showed a significant fall in FBS and an increase in body weight. However, the transfer latency in PA, 24 hours after the aversive shock was decreased compared with SR+CT+EE group, suggesting no improvement in avoidance learning and memory. Insulin-treated animals spent lesser time in target quadrant of MWM, indicating reduced spatial learning and memory compared to those treated with SR+CT+EE. They also showed less number of entries and spent lesser time in OA of EPM, suggesting no significant reduction in anxiety levels. Thus, the beneficial effect of SR+CT+EE was demonstrated in cognitive and behavioral parameters in EPM, PA and MWM tests, compared with conventional therapy.
In congruence with the findings of the present study, many researchers demonstrated the beneficial effect of combined herbal therapy. A polyherbal therapy of Angelica gigas, Saururus chinensis and Schizandra chinensis, showed neuroprotective activity in Alzheimer’s disease, stroke, ischemic injury and other neurodegenerative diseases [38]. Ginkgo biloba, another neuroprotective agent, is often prescribed in combination with Panax ginseng to obtain traditional synergistic effects [39]. Combined administration of vitamin C and E for 30 days from the day one of onset of diabetes alleviated the negative influence of diabetes on learning and memory.
The results of the present study demonstrated the beneficial effects of the combination of alcoholic extracts of roots of Salacia reticulata, Clitoria ternatea, with rearing in an enriched environment (SR+CT+EE) compared to insulin on diabetes-induced behavioral and cognitive changes in juvenile rats. The effect found to be better than insulin, a conventional treatment for T1DM. After treatment with SR+CT+EE for 30 days, diabetic animals were able to attain not only the normoglycemic levels but also showed significantly improved measures in almost all three tests (EPM, PA, and MWM), suggesting the recovery of deficits in cognition and behavioral changes. In earlier studies, authors have shown the beneficial effect of SR and CT separately [40].
The novel, non-invasive cognitive enhancers that are developed in the study may prove beneficial in other conditions, such as neurological disorders in children or dementia in old age or for cognition enhancement in the healthy younger population of course with slight modifications as per the pattern of neurodegeneration. If potent cognitive enhancers are available in the future, they are likely to have a substantial economic impact. Although the results of the present study are in support of the above conclusions, further studies are clearly warranted to firmly establish the link between hyperglycemia and cognitive deficits. There is a need for advanced techniques and molecular studies to explore underlying mechanisms of diabetic encephalopathy in T1DM of early onset in particular. The present study necessitates the identification of active fractions or phytochemical constituents with their mode of actions of some more herbs with nootropic activity.
Citation: Rajashree R, Khlokute SD, Goudar SS (2019) Effect of Enriched Environment on the Cognitive and Behavioural Changes in the Streptozotocin Induced Young Diabetic Rats. Anat Physiol 9: 1000316
Received: 22-May-2019 Accepted: 19-Jul-2019 Published: 26-Jun-2019 , DOI: 10.35248/2161-0940.19.9.316
Copyright: © 2019 Rajashree R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.