
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Research Article - (2024)Volume 10, Issue 1
Objective: By dissecting systemic inflammatory response, we aimed to provide the reference to treatment for pediatric patients with sepsis.
Methods: 62 pediatric children with sepsis and 48 pediatric children without sepsis were enrolled in study. HLA- DR/CD14+ expression, proportion of CD4+CD25+ Forkhead box protein P (Foxp3+) Treg cells and IL-27+, CD4+ cells were analyzed by flow cytometry. Foxp3, CTLA-4, GITR, IL-10, L-17A, IL-17F and IL-27 mRNA levels were evaluated by real-time PCR. IL-4, IFN-γ and TGF-β were measured by enzyme-linked immunosorbent assay.
Results: Compared to children without sepsis, there are higher level of IFN-γ, IL-17A, IL-17F, IL-27 mRNA, Foxp3, CTLA-4, IL-10 mRNA and proportion of CD4+, IL-27+ cells and lower of TGF-β and CD4+CD25+Treg cells in children with sepsis. The HLA-DR was significantly lower in sepsis group, but greater than 30%.
Conclusion: Pro-inflammatory response dominates at early stage in sepsis children. Therefore, removing inflammatory response rather than immunomodulation is key of treatment for pediatric sepsis.
Pediatric patient; Sepsis; Systemic inflammatory responses; Compensatory anti-inflammatory responses; Regulatory T cells; IL-27
HLA-DR: Human Leukocyte Antigen-Death Receptor; PCR: Polymerase Chain Reaction; CTLA-4: Cytotoxic T-Lymphocyte-Associated Protein 4; GITR: Glucocorticoid—Induced Tumor Necrosis Factor Receptor; IL-10: Interleukin-10; IL-17: Interleukin-17; IL-27: Interleukin-27; mRNA: messenger Ribonucleic Acid; IFN-γ: Interferon-γ; IL-4: Interleukin-4; TGF-β: Transforming Growth-Factor; CD: Cluster of Differentiation; SIRS: Systemic Inflammatory Response Syndrome; CARS: Compensatory Anti-Inflammatory Response Syndrome
Sepsis is a leading cause of death in children with serious diseases, with its high morbidity and mortality in pediatric patients, sepsis aroused the worldwide consideration in modern critical care medicine [1,2].
Sepsis occurs as a generalized inflammatory cascade including severe sepsis, septic shock and multi-organ failure, the overwhelming or persistent release of pro-inflammatory mediators from Systemic Inflammatory Response Syndrome (SIRS) and anti- inflammatory mediators from Compensatory Anti-Inflammatory Response Syndrome (CARS) result in inflammatory imbalance and immunologic dissonance [3,4]. Researches indicate patient’s prognosis related to the inflammatory response and immune status in early stage, so early detection and treatment of sepsis is vital to prevent widespread tissue injury, morbidity [5].
The dissonant immune response in sepsis involves abnormal Cluster of Differentiation (CD4) and (CD8) T cell response [6]. CD4 T cell plays important role in primary CD8 T cell response, formation of functional CD8 T cell memory and efficient isotype switching of B cells [7]. By producing cytokines, activating and altering the migration of other immune cells, CD4 T cell can effectively respond to infection. The activation of Type 1 T helper (Th1) CD4 cells results in the production of cytokines, such as Interleukin 2 (IL-2) and Interferon-γ (IFN-γ), IL-10, which in turn activates macrophages. While the activated Th2 CD4 cells results in various different immune responses by producing cytokines such as IL-4, IL-5 and IL-13 [8]. Therefore, the observation of T cell immunity at early stage conduces to understand the regulation of the immune status and identify novel strategies in pediatric sepsis patient.
As a member of IL-12 family of cytokines, IL-27 signals through a heterodimeric receptor complex consisting of the common IL-6 receptor chain Glycoprotein 130 (gp 130) and a unique IL-27 Receptor α chain (IL-27R) WSX-1 [9]. By acting on various immune cells including T cells, B cells, macrophages and dendritic cells, IL- 27 presents as pleiotropic cytokine in both pro-inflammatory and anti-inflammatory [10,11]. Studies has suggested that IL-27 could be a diagnostic biomarker for predicting bacterial infection in sepsis patients and the blocking of IL-27 function could be used as a therapeutic strategy of sepsis [12-14].
Human Leukocyte Antigen DR (HLA-DR), a glycosylated cell surface transmembrane protein expressed on antigen-presenting cell monocytes HLA-DR, (mHLA-DR), is essential for activation of CD4 or CD8 T cells to initiate the specific immune response [15,16]. mHLA-DR is considered as a reliable biomarker in critical infection patients, a lower HLA-DR than 30% means that patient’s immune function is suppressed [17-19]. Evidences indicate the mortality increased 30-fold when mHLA-DR expression reduced by 30% [20,21].
Currently, few of biomarkers had been evaluated for sepsis to different potential sources of infections [22,23]. In this study, we are aiming to observe the early immune status in pediatric patients with sepsis and the performance of several biomarkers, by which provides the reference to the diagnosis and treatment to pediatric patients with sepsis.
Patients
62 pediatric patients with sepsis in the Pediatric Intensive Care Unit (PICU) of Guizhou Provincial People's Hospital between January 2015 and May 2017 were studied as sepsis group, including 42 boys and 20 girls aged from 2 to 13 years (mean 6.27 years). The diagnosis of sepsis was conducted according to the recommended criteria for pediatric sepsis [24]. Another 48 pediatric patients consisted of 36 boys and 12 girls aged from 2 months to 14 years (mean 5.75 years) without sepsis, who underwent surgery (foreskin circumcision: 32; non-incarcerated hernia: 16) in the pediatric surgery department, were studied as control group. Pediatric patients with autoimmune diseases, immune deficiency, genetic metabolic disorders and tumor were excluded from this study. Pediatric patients who accepted the treatments with drug or blood products that influence the immune system or died within 24 hours after admission were excluded as well.
The diagnostic criteria of sepsis
Sepsis was defined as the life-threatening organ dysfunction caused by a dysregulated host response to infection according to the sepsis-3 criteria: increase in sequential organ failure assessment score by ≥ 2 at day 1 and suspicion of infection.
Blood sample collection
Venous blood was immediately obtained after admission in hospital or diagnosis of sepsis. 1 ml of blood was used for the detection of various inflammatory or anti-inflammatory factors in serum and 8 ml was used for detection of cell sorting, analysis and gene expression.
The detection of pro/anti-inflammatory cytokines and HLA-DR
The HLA-DR expression on CD14+ monocytes, the proportion of CD4+CD25+Foxp3+ regulatory T cells (Treg cells) and CD4+ IL-27+ cells were analyzed by flow cytometry, the corresponding antibodies used in this study are anti-IL-27 EBI3 PE antibody (eBioscience, CA, USA), anti-CD4 Peridinin Chlorophyll Protein complex (PerCP) antibody, anti-CD25 Fluorescein Isothiocyanate (FITC) antibody and anti-Foxp3 Phycoerythrin (PE) antibody (BD Biosciences, San Jose, CA, USA) respectively and the isotype control antibodies (eBioscience, CA, USA). The Foxp3, CTLA- 4, GITR, IL-10, L-17A, IL-17F and IL-27 mRNA levels in CD4+ cells were evaluated by real-time PCR, the primers used in the RTPCR primers sequences shown in supplementary materials. And cytokines IL-4, IFN-γ and TGF-β were measured by enzyme-linked immunosorbent assay with Enzyme-Linked Immunosorbent Assay (ELISA) kits (CUSABIO Wuhan, China).
Statistical analysis
Data was presented as mean ± Standard Deviation (SD). Software SPSS 17.0 (SPSS Inc., USA) was utilized in the current study. Differences between groups were analyzed by two-tailed t test or mann–whitney test. P ≤ 0.05 was considered as statistically significant and p<0.01 was considered as remarkably statistically significant.
Pro-inflammatory responses at an early stage of sepsis: The differential expression of selected pro-inflammatory factors was detected: the serum level of IFN-γ (99.29 ± 16.01) was significantly higher in patients with sepsis than that in control group (55.39 ± 8.35) (p< 0.01). And the transcription levels of IL-17A and IL- 17F in CD4+T cells of patients with sepsis were significantly higher than that in control group (p< 0.01) (Table 1).
| Items | Patients (n=62) X ± Standard Deviation (SD) |
Control (n=48) X ± Standard Deviation (SD) |
p-value |
|---|---|---|---|
| Interleukin-4 (IL-4) pg/ml | 103.57 ± 20.27 | 113.32 ± 17.68 | >0.05 |
| Transforming Growth-Factor (TGF-β) pg/ml | 1139.45 ± 161.82 | 1772.76 ± 397.79 | <0.05 |
| Interferon -γ (IFN-γ) pg/ml | 99.29 ± 16.01 | 55.39 ± 8.35 | <0.01 |
| Interleukin-4 messenger Ribonucleic acid (IL-10mRNA) | 1.45 ± 0.59 | 0.38 ± 0.09 | <0.01 |
| Interleukin-17A messenger Ribonucleic acid (IL-17A mRNA) | 1.03 ± 0.11 | 0.26 ± 0.04 | <0.01 |
| Interleukin-17F messenger Ribonucleic acid (IL-17F mRNA) | 0.46 ± 0.12 | 0.09 ± 0.01 | <0.01 |
| Interleukin-27 messenger Ribonucleic acid (IL-27 mRNA) | 0.36 ± 0.28 | 0.09 ± 0.02 | <0.01 |
| Forkhead Box Protein 3 (FOXP3) mRNA | 0.942 ± 0.359 | 0.518 ± 0.112 | <0.01 |
| Cytotoxic T-Lymphocyte–Associated Antigen 4 (CTLA-4) mRNA | 1.15 ± 0.26 | 0.52 ± 0.11 | <0.05 |
| Glucocorticoid-Induced TNFR (Tumor Necrosis Factor Receptor )-Related (GITR) mRNA | 0.29 ± 0.16 | 0.26 ± 0.01 | >0.05 |
| Cluster of Differentiation (CD4+) Interleukin-27(IL-27+) Regulatory T cells (Treg) (%) | 5.44 ± 1.04 | 1.51 ± 0.43 | <0.01 |
| CD4+CD25+FOXP3+Treg (%) | 6.72 ± 2.04 | 12.88 ± 2.61 | <0.01 |
| Human Leukocyte Antigen-DR (HLA-DR+) expression rate (%) | 75.36 ± 6.77 | 95.13 ± 2.69 | <0.01 |
Note: The serum contents of cytokines (Interleukin-4 (IL-4), Transforming Growth-Factor (TGF-β) and Interferon-γ (INF-γ)) were measured. The mRNA levels (IL-10, L-17A, IL-17F, IL-27, Foxp3, CTLA-4 and GITR) were measured in sorted CD4+ cells. The percentages of CD4+IL-27+ cells or CD4+CD25+FOXP3+Treg cells were calculated within CD4+ cell population. The HLA-DR+ expression rate was calculated within CD14+ monocyte population. The data were presented as Mean ± Standard Deviation (x ± SD).
Table 1: The comparison of pro/anti-inflammatory factors and immune status in pediatric patients with or without sepsis.
The expression of HLA-DR on the surfaces of CD14+ monocytes was also detected. It was 75.36 ± 6.77 in patients with sepsis, significantly lower than that of control (95.13 ± 2.69) (p< 0.01). But both were higher than 30% (Table 1 and Figure 1).
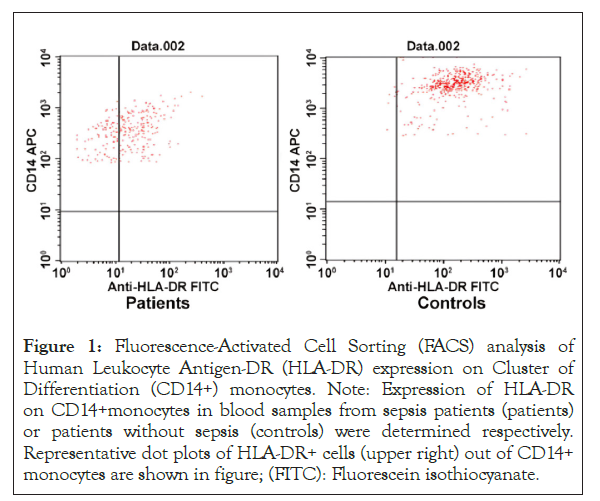
Figure 1: Fluorescence-Activated Cell Sorting (FACS) analysis of Human Leukocyte Antigen-DR (HLA-DR) expression on Cluster of Differentiation (CD14+) monocytes. Note: Expression of HLA-DR on CD14+monocytes in blood samples from sepsis patients (patients) or patients without sepsis (controls) were determined respectively. Representative dot plots of HLA-DR+ cells (upper right) out of CD14+ monocytes are shown in figure; (FITC): Fluorescein isothiocyanate.
Anti-inflammatory responses at early stage of sepsis: The differential expressions of selected markers of the anti-inflammatory responses were detected. The serum level of Transforming Growth-Factor (TGF-β) (1139.45 ± 161.82) was significantly lower in patients with sepsis than that in control (1772.76 ± 397.79) (p<0.05). However, there was no significant difference between sepsis patients and control regarding to IL-4 serum level (Table 1). The peripheral CD4+CD25+Foxp3+Treg ratio in patients with sepsis was significantly lower than that in controls (p<0.01) (Figure 2 and Table 1). However, compared to patients without sepsis, the expression of Treg specific genes in CD4+T cells, Foxp3, CTLA-4 and IL-10 were significantly higher in patients with sepsis (p<0.01). There was no significantly difference of mRNA level of GITR in CD4+T between in sepsis group and control group (Table 1).
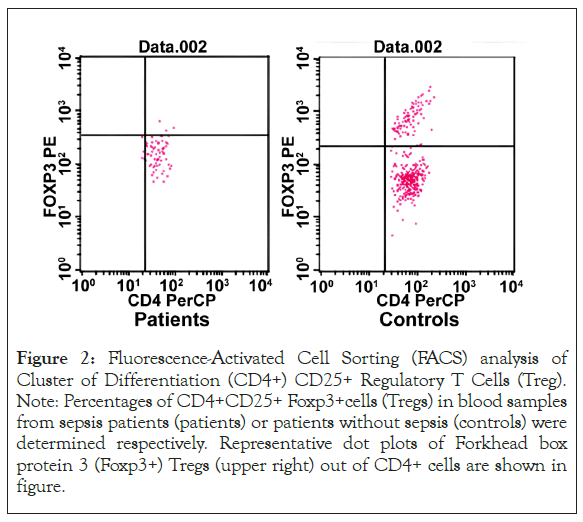
Figure 2: Fluorescence-Activated Cell Sorting (FACS) analysis of Cluster of Differentiation (CD4+) CD25+ Regulatory T Cells (Treg). Note: Percentages of CD4+CD25+ Foxp3+cells (Tregs) in blood samples from sepsis patients (patients) or patients without sepsis (controls) were determined respectively. Representative dot plots of Forkhead box protein 3 (Foxp3+) Tregs (upper right) out of CD4+ cells are shown in figure.
IL-27 expression at early stage of sepsis: IL-27 gene expression in CD4+T cells was higher in patients of sepsis comparing to that in control group (p<0.01). Meanwhile, the ratio of CD4+IL-27+ cells was significantly higher in patients with sepsis comparing to that in control group (p<0.01) as well (Figure 3 and Table 1).
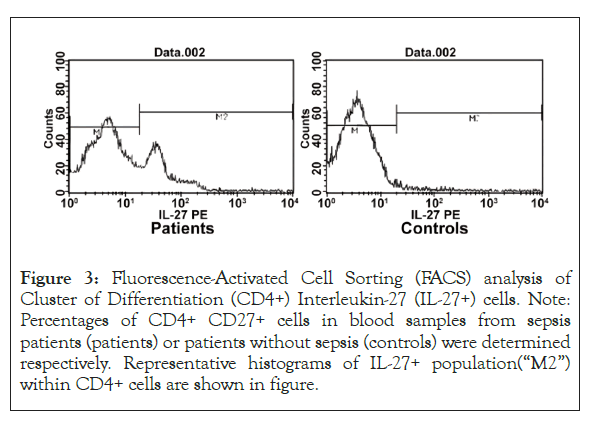
Figure 3: Fluorescence-Activated Cell Sorting (FACS) analysis of Cluster of Differentiation (CD4+) Interleukin-27 (IL-27+) cells. Note: Percentages of CD4+ CD27+ cells in blood samples from sepsis patients (patients) or patients without sepsis (controls) were determined respectively. Representative histograms of IL-27+ population(“M2”) within CD4+ cells are shown in figure.
The pathogenic mechanism of sepsis involves the systemic inflammatory network, gene polymorphisms and immune dysfunction, systemic inflammation and immune status of patient with sepsis determine the progression of sepsis [25]. In the current study, both pro-inflammation and anti-inflammation factors in the serum, the status of CD4+CD25+ Treg cells were analyzed in pediatric patients with and without sepsis. Results indicate the mixed immune response presented at early stage in pediatric sepsis patients, IL-27 may exert bi-directional regulation in early immune responses, an elaborate regulation of immune status regarding on IL-27 expression can be used as an effective therapeutic strategy in of pediatric sepsis patients.
HLA-DR molecules are central to the specific immune response, the decreasing of HLA-DR on monocytes connects with complications of infections and progression of sepsis [26]. And reduction by 30% of HLA-DR expression can be considered as immune suppression [27]. In this study, the expression of HLA-DR on CD14+ monocytes in sepsis patients is lower than that in control group, but both are higher than 30%, which suggests that although the early immune function is stimulated by the inflammatory response, it is not suppressed (Table 1).
Comparing to patients without sepsis, the pro-inflammatory factor IFN-γ in serum, IL-17A / IL-17F secreted by Th17 cells, the ratio IFN-γ/ IL-4 in pediatric sepsis patients significantly increased, while the anti-inflammatory factor TGF-β significant decreased. These results indicate that both the pro- and anti-inflammatory responses presented at early stage in pediatric sepsis patients, the pro-inflammatory response dominants. In this study, we also investigated the CD4+CD25+Treg ratio and the expression of specific genes Foxp3, CTLA-4, GITR and IL-10 in CD4+ T cells. Although no significant difference shown in GITR, while the transcription levels in CD4+ T cells of Foxp3, CTLA-4 and IL- 10 are significant higher in sepsis patients than those in controls, combination with the results of HLA-DR expression on CD14+ monocytes, our studies suggest the compensatory anti-inflammatory responses presented in children with sepsis at early stage. The immune disorder in sepsis is caused by a dynamic alteration between Systemic Inflammatory Response Syndrome (SIRS) and (Compensatory Anti-Inflammatory Response Syndrome) CARS and the anti-inflammatory factors released by CARS are protective reaction to SIRS, which led to complicated immune process and imbalanced immune response [28]. Systematic surveillance and evaluation of the immune status of patients are vital to early sepsis patients. Combination with above the results of HLA-DR expression, this study implies that the therapeutic strategies at early stage in pediatric patient with sepsis should base on the effective treatment to pro-inflammatory reaction and suppressing the damage resulting from the immune response to infection.
IL-27, as a new member of IL-12 family, plays duel-functions in the immune and inflammation reactions [29]. IL-27 exerts duel-regulation effects on Th1 cells [30]. Though IL-27 cannot induce the production of Foxp3+ Treg, it can promote Tr1 cell development and differentiation through IL-10 [31,32]. While IL- 10 participates in IL-17 suppression by IL-27 and prevents Foxp3+ Treg cells differentiating from CD4+ T through down-regulating IL-6 and TGF-β expression. Therefore, IL-27 plays a critical role in balancing the immune response. In addition, IL-27 can also induce Major Histocompatibility Complex (MHC) molecule expression in non-immune cells to endow them with the ability to perform antigen presentation. In the current study, the increased expression of IL-27, IFN-γ, IL-17A, IL-17F and IL-10 in CD4+T cells were observed in the pediatric sepsis at early stage. The serum level of TGF-β and ratio of CD4+, CD25+ and Treg cells in patients were lower in sepsis patients than that in controls. These findings imply that IL-27 might promote the release of IFN-γ and IL-17, repress the TGF-β expression and differentiation of Treg cells. Meanwhile, IL-27 might increase the expression of IL-10, Foxp3+ and CTLA-4, by which could enhance the immune suppression effect of Treg and balance immune response. Therefore, by the duel effects on the immune system, IL-27 could be used as a novel target for the treatment of pediatric sepsis, but how to utilize its advantages remains to be further studied.
In sum, our results collectively show that both pro-inflammation and anti-inflammation response presented at early stage in pediatric sepsis patients, while pro-inflammation response predominate in this stage. Although the immune status of pediatric patients with sepsis declined in early stage of sepsis, it was not immunosuppressed, therefore, we should focus on the treatment of anti-inflammation rather than the enhancement of immune at early stage. As a duel-regulation cytokine, IL-27 balances the duel immune response by regulating the differentiation of immune cells and secretion of other cytokines in early stage of pediatric sepsis patients and can be used as a potential therapeutic strategy for pediatric sepsis patients. However, in consideration of limitations of this study, several aspects should be further discussed and explored in future. Firstly, the immuno-logical system is influenced by age-related factors, immune status in individual pediatric patients should be further individually analyzed. Secondly, our conclusions about inflammatory response based on the cytokines we examined in this study, more classic inflammatory cytokines should be detected to confirm the inflammatory response status in early stage of pediatric sepsis patients.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Li C, Lv X, Fan X, Huang D, Li J (2024) Early Mixed Inflammatory Response in Children with Sepsis. Immunotherapy (Los Angel). 10:247.
Received: 31-Jan-2024, Manuscript No. IMT-24-29496; Editor assigned: 02-Feb-2024, Pre QC No. IMT-24-29496 (PQ); Reviewed: 16-Feb-2024, QC No. IMT-24-29496; Revised: 23-Feb-2024, Manuscript No. IMT-24-29496 (R); Published: 01-Mar-2024 , DOI: 10.35248/2471-9552.24.10.247
Copyright: © 2024 Li C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.