Lupus: Open Access
Open Access
ISSN: 2684-1630
ISSN: 2684-1630
Research - (2019)Volume 4, Issue 1
Cardiovascular disease (CVD) is a major complication of systemic lupus erythematosus (SLE) and is now a leading cause of death for these patients. In this study, 23 SLE patients asymptomatic for CVD underwent a comprehensive echocardiographic examination to detect subclinical cardiac involvement. According to their SELENA-SLEDAI score, they were divided into two groups: SELENA-SLEDAI ≤ 12 (n=13, 12 females) and SELENA-SLEDAI >12 (n=10, all females), indicative of mild to moderate and severe SLE, respectively. Patients in the latter group had significant increases in left ventricular (LV) mass, LV end-diastolic volume, left atrial volume and right heart parameters (pulmonary arterial pressure, tricuspid regurgitation velocity and diameter of the inferior cava) compared to the mild-moderate group. Diastolic dysfunction, evaluated as the early/late (E/A) and early/septal velocity (E/e’) ratios, was not detected in either group. The Framingham scores of all patients correlated directly with LV mass and indirectly with both the E/A ratio, thus evidencing subclinical myocardial involvement in patients with severe SLE. Overall, our results demonstrate the presence of early-stage, and thus clinically silent, diastolic dysfunction in patients with severe SLE. This study underscores the importance of echocardiography for the early detection of LV diastolic dysfunction as it may progress to global systolic dysfunction. Echocardiography should therefore be included in the routine examination of SLE patients.
Systemic lupus erythematosus; Cardiovascular disease; Echocardiography; Left ventricular diastolic dysfunction
Systemic lupus erythematosus (SLE) is a chronic and multifaceted autoimmune disease that mostly affects young women [1]. Although the survival of SLE patients has remarkably improved in the last several years, cardiovascular disease (CVD) is being diagnosed with increasing frequency and is currently detected in up to 50% of SLE patients. All cardiac structures may be involved, including the pericardium, endocardium, myocardium, coronary arteries and conductive tissue [2]. Compared to the general population, SLE patients have a 2 to 9- fold higher risk of cardiovascular events, such as myocardial infarction, stroke and heart failure [3-5]. The mechanisms underlying CVD in SLE are multifactorial and still not well understood. Traditional risk factors, such as hypertension, smoking, sedentary lifestyle and elevated lipid levels, do not fully explain the higher risk of CVD development in SLE, and SLE itself is an independent risk factor for CVD [6]. SLEspecific factors that play an additional role include disease activity, persistence of systemic and local inflammation, immune abnormalities, an accelerated atherosclerotic process, antiphospholipid antibodies (anti-cardiolipin and anti-β2 glycoprotein I) and potential adverse effects of therapies, especially glucocorticoids (GCs) and nonsteroidal anti-inflammatory drugs [7-11].
Given this complex interplay between traditional and SLE-specific risk factors, standard risk calculators such as the Framingham equations, the Atherosclerotic Cardiovascular Disease Risk Algorithm (ASCVD), and Systematic Coronary Risk evaluation (SCORE) underestimate the risk of CVD in the context of SLE [12,13]. Cardiac abnormalities, especially left ventricular (LV) diastolic dysfunction, are common and persistent findings in SLE patients and may be the only manifestation of cardiac involvement preceding global systolic dysfunction [14].
In this study, we examined the echocardiographic parameters in SLE patients to assess the CVD risk. Our results point to an increased LV mass and left atrial (LA) volume as early markers of diastolic dysfunction in SLE patients.
Twenty-three SLE patients attending our Internal Medicine unit from January to December 2017 were included in the study. All patients fulfilled the American College of Rheumatology’s diagnostic criteria for SLE [15]. Patients completed a questionnaire and underwent a comprehensive physical examination. Blood samples obtained from all patients were analyzed for the following: complete blood count, low/high density lipoproteins, triglycerides and antidouble- stranded DNA (anti-dsDNA) antibodies. Additional laboratory tests included erythrocyte sedimentation rate, C-reactive protein level, estimated glomerular filtration rate, as well as uric acid and complement (C3, C4) levels. The Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) version of the SLE Disease Activity Index (SLEDAI) score [16] was adopted to determine SLE disease activity. All patients were evaluated by standard transthoracic echocardiography. Demographic characteristics as well as clinical and laboratory findings were recorded. Patients with any congenital or acquired CVD event, any systemic disease (endocrine, metabolic or infectious), hypertension, diabetes mellitus, chronic kidney diseases or chest disease were excluded. All patients provided written informed consent. The study protocol was approved by the Ethics Committee of the University of Bari Medical School and conformed to the good clinical practice guidelines of the Italian Ministry of Health and the ethical guidelines of the Declaration of Helsinki, as revised and amended in 2004.
Echocardiography
All echocardiographic measurements were assessed using an Esaote ultrasound machine (MyLab™Seven) with a 2.5-MHz electronic transducer. The patients were examined in the left lateral decubitus position. From the parasternal log-axis view, B-mode and M-mode echocardiography was used to obtain end-diastole measurements of interventricular septum thickness, LV posterior wall diameter, and interior LV diameter (Figures 1A and 1B). LV mass was determined using the Devereux and Reichek "cube" formula [17]. Relative wall thickness was calculated using an internationally validated formula [18]. An apical four-chamber (A4C) view was used to evaluate the tricuspid annular plane systolic excursion, right atrial area, LV enddiastolic volume, LV ejection fraction, and LA volume (Figures 1C-1F). A subcostal view was used to measure the diameter of the inferior vena cava (IVC). Aortic root diameter, LA volume and LV mass were indexed to body surface area.
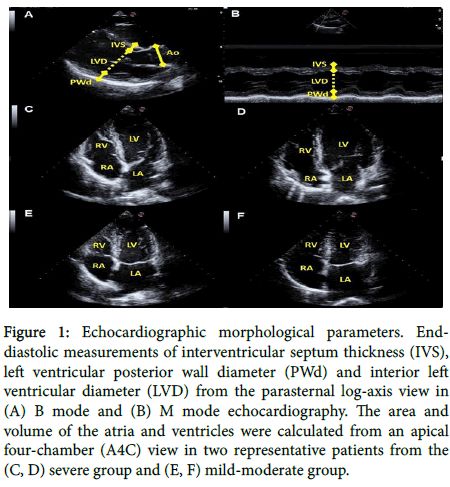
Figure 1. Echocardiographic morphological parameters. Enddiastolic measurements of interventricular septum thickness (IVS), left ventricular posterior wall diameter (PWd) and interior left ventricular diameter (LVD) from the parasternal log-axis view in (A) B mode and (B) M mode echocardiography. The area and volume of the atria and ventricles were calculated from an apical four-chamber (A4C) view in two representative patients from the (C, D) severe group and (E, F) mild-moderate group.
Aortic root diameter and Doppler measurements were obtained using B-mode echocardiography and a parasternal log-axis view. Tricuspid regurgitation velocity (TRV) was estimated by measuring the maximum velocity of the regurgitation jet using continuous-wave Doppler and an A4C view (Figure 2A). The peak value was then used to calculate the pressure difference between the right ventricle and right atrium according to the simplified Bernoulli equation (p=4 [TRVmax]2). The estimated TRV was then used to estimate pulmonary arterial pressure as a derivative sum with the right atrial filling pressure (estimated based on the size and respiratory collapsibility of the IVC during normal respiration) and the tricuspid regurgitation pressure. Pulsed-wave Doppler was used to detect the peak velocity during early (E) and late (A) diastole (Figure 2B). The septal velocity (e’) was measured using tissue Doppler (Figure 2C) or pulsed-wave Doppler (Figure 2D) imaging. Diastolic dysfunction was diagnosed as an E/A ratio <1 or >2 and/or an E/e’ ratio >15.
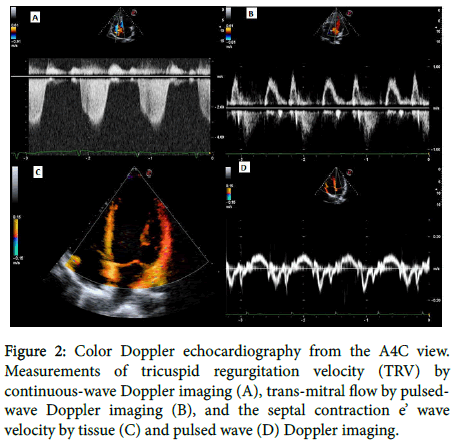
Figure 2. Color Doppler echocardiography from the A4C view. Measurements of tricuspid regurgitation velocity (TRV) by continuous-wave Doppler imaging (A), trans-mitral flow by pulsedwave Doppler imaging (B), and the septal contraction e’ wave velocity by tissue (C) and pulsed wave (D) Doppler imaging.
Statistical analysis
All statistical analyses were carried out using the Prism program (Graph Pad Software). The distribution of dichotomous values was analyzed using a chi-squared test. Non-normally distributed data were analyzed using non-parametric statistics. The Mann-Whitney test was used for comparisons of groups and the Spearman rank test for correlations. Normally distributed data were analyzed using parametric statistics; group comparisons were carried out using unpaired t tests. p-values are shown only for comparisons in which the differences were statistically significant, defined as p values <0.05.
The demographic and clinical characteristics of the study patients are summarized in (Table 1). The 22 female patients (96%) and 1 male patient (4%) had a mean age of 43.7 ± 11.9 years and a disease duration of 8.9 ± 10.4 years. At the time of analysis, 65.2% of the patients were being treated with GCs; the mean daily oral dose of prednisone or prednisone equivalent was 9.2 ± 4.2 mg/day. In addition, 39.1% of patients were being treated with disease-modifying anti-rheumatic drugs, including methotrexate, sulfasalazine and leflunomide; 21.7% with antimalarials; 13% with mycophenolate mofetil; and 17.4% with belimumab. An additional subgroup of 26.1% patients was not receiving treatment at the time of the study. All patients had a normal body mass index and body surface area. Systolic and diastolic blood pressures were also normal. All patients were negative for lupus anticoagulant or anti-phospholipid antibody syndrome, none were diabetic, and four were smokers.
| Demography | |
|---|---|
| Age (years) | 43.7 ± 11.9 |
| Females/Males | 22/1 |
| Clinical | |
| Duration of SLE (years) | 8.9 ± 10.4 |
| SELENA-SLEDAI score | |
| SELENA-SLEDAI ≤ 12 (mild-to-moderate) | 5.5 ± 3.1 |
| SELENA-SLEDAI >12 (severe) | 20.4 ± 8.8 |
| Therapy | |
| Steroids | 15 (65.2) |
| DMARDs | 10 (39.1) |
| Antimalarials | 5 (21.7) |
| Mycophenolate | 4 (13.0) |
| Belimumab | 6 (17.4) |
| No treatment | 6 (26.1) |
| Smoker (yes/no) | 4/19 |
| SBP (mmHg) | 117.8 ± 15.7 |
| DBP (mmHg) | 70.9 ± 10.9 |
| BMI (kg/m2) | 24.2 ± 4.6 |
| BSA (m2) | 1.7 ± 0.2 |
Table 1: Baseline characteristics of 23 SLE patients.
Based on the SELENA-SLEDAI score, the patients were grouped into two groups, SELENA-SLEDAI ≤ 12 and SELENA-SLEDAI >12, corresponding to mild-moderate and severe disease, respectively [19]. The 12 females and 1 male in the mild-moderate group had a mean age of 44.36 ± 14.40 years. All 10 patients in the severe group were female; their mean age was 41.75 ± 8.57 years (Table 2A).
| Variables | Mild-to-moderate | Severe | p value |
|---|---|---|---|
| (n=13) | (n=10) | ||
| Age | 44.36 ± 14.40 | 41.75 ± 8.57 | ns |
| SELENA-SLEDAI score | 5.5 ± 3.1 | 20.4 ± 8.8 | <0.0001 |
| WBC (× 103cell/mm3) | 6.5 ± 2.8 | 4.5 ± 2.4 | ns |
| Neutrophils (× 103 cell/mm3) | 3.8 ± 2.6 | 2.6 ± 2.2 | ns |
| Lymphocytes (× 103 cell/mm3) | 1.19 ± 1.04 | 0.85 ± 0.65 | ns |
| Hb (gr/dl) | 11.5 ± 1.89 | 11.3 ± 1.55 | ns |
| PLT (× 103 cell/mm3) | 260.2 ± 165.7 | 210.8 ± 106.5 | ns |
| ESR (mm/hour) | 59.0 ± 39.5 | 37.3 ± 26.3 | ns |
| CRP (mg/dl) | 12.1 ± 11.3 | 8.1 ± 1.4 | ns |
| eGFR (ml/h) | 98.1 ± 29.5 | 99.5 ± 21.5 | ns |
| Anti-dsDNA (UI/l) | 39.5 ± 21.1 | 838.0 ± 523.8 | 0.03 |
| C3 (mg/dl) | 0.99 ± 0.45 | 0.60 ± 0.35 | 0.04 |
| C4 (mg/dl) | 0.21 ± 0.1 | 0.10 ± 0.07 | 0.006 |
Table 2A: Clinical and laboratory findings in mild-to-moderate and severe SLE patients.
A comparison of the clinical and laboratory parameters of the two groups showed a significantly higher mean SELENA-SLEDAI score (5.5 ± 3.1 vs. 20.4 ± 8.8; p <0.0001) and mean anti-dsDNA antibody titer (39.5 ± 21.1 vs. 838.0 ± 523.8; p=0.03) in the severe than in the mild-moderate group. Patients with severe disease also had significantly lower mean serum C3 (0.99 ± 0.45 vs. 0.60 ± 0.35, p=0.04) and C4 (0.21 ± 0.1 vs. 0.10 ± 0.07; p=0.006) levels. The mean values of all other parameters did not significantly differ between the two groups (Table 2A).
There were no significant differences between the two groups with respect to traditional CVD risk factors such as the Framingham score, including any of its components [age, sex, presence of hypertension, smoking habit, total cholesterol, low density lipoproteins, systolic blood pressure]. The differences in the high-density lipoprotein level, uric acid level, and diastolic blood pressure were also not significant (Table 2B). However, the mean triglyceride level (122.0 ± 33.2 vs. 198.4 ± 96.0; p=0.025) was significantly higher in the severe group (Table 2B).
| Variables | Mild-to-moderate | Severe | p value |
|---|---|---|---|
| (n=13) | (n=10) | ||
| Age | 44.36 ± 14.40 | 41.75 ± 8.57 | ns |
| Framingham score | 4.3 ± 3.8 | 3.9 ± 3.7 | ns |
| Sex (F/M) | 12/1 | 10/0 | ns* |
| Smoker (yes/no) | 1/12 | 3/7 | ns* |
| Cholesterol (mg/dl) | 183.5 ± 35.0 | 189.8 ± 52.2 | ns |
| LDL (mg/dl) | 105.1 ± 29.9 | 104.8 ± 36.9 | ns |
| HDL (mg/dl) | 53.8 ± 14.7 | 48.8 ± 19.7 | ns |
| Triacilglycerols (mg/dl) | 122.0 ± 33.2 | 198.4 ± 96.0 | 0.025 |
| Uric acid (mg/dl) | 3.6 ± 1.1 | 4.0 ± 1.1 | ns |
| SBP (mmHg) | 117.3 ± 11.3 | 111.3 ± 17.9 | ns |
| DBP (mmHg) | 72.27 ± 8.17 | 65.63 ± 12.37 | ns |
Table 2B: Analysis of the traditional CVD risk factors in mild-to-moderate and severe SLE patients.
Asymptomatic cardiac hypertrophy, defined according to recent ASE/EACVI guidelines [20], was detected in both groups although none of the patients had hypertension, as per the exclusion criteria. Echocardiography revealed a greater absolute (169.3 ± 34.6 g vs. 215.4 ± 61.5 g, p=0.048) and indexed (101.8 ± 15.6 g/m2 vs. 133.3 ± 45.1 g/m2, p=0.045) LV mass in the severe group, suggesting a worsening of cardiac involvement in these patients (Table 3). Further support for this finding was obtained by analyzing the single parameters included in the Devereux formula for LV mass determination: LV diameter, interventricular septum thickness, and posterior wall diameter. LV diameter was higher, with a trend toward statistical significance, in the severe than in the mild-moderate group, whereas interventricular septum thickness and posterior wall diameter were similar.
| Variables | Mild-to-moderate | Severe | p value |
|---|---|---|---|
| (n=13) | (n=10) | ||
| LVM (gr) | 169.3 ± 34.6 | 215.4 ± 61.5 | 0.048 |
| LVMi (gr/m2) | 101.8 ± 15.6 | 133.3 ± 45.1 | 0.045 |
| LVD (mm) | 44.9 ± 4.0 | 50.2 ± 7.5 | 0.06 |
| IVS (mm) | 10.5 ± 0.7 | 11.1 ± 2.1 | ns |
| PWd (mm) | 10.4 ± 0.9 | 11.2 ± 2.0 | ns |
| RWT | 0.44 ± 0.4 | 0.45 ± 0.1 | ns |
| LVEDV (ml) | 84.3 ± 15.3 | 105.9 ± 23.9 | 0.028 |
| EF (%) | 63.4 ± 4.2 | 60.8 ± 3.0 | ns |
| Ao (mm) | 29.2 ± 3.0 | 29.1 ± 3.3 | ns |
| Aoi (mm/m2) | 17.7 ± 1.9 | 17.6 ± 3.3 | ns |
| LAV (ml) | 47.5 ± 14.9 | 65.4 ± 20.1 | 0.039 |
| LAVi (ml/m2) | 28.5 ± 8.4 | 39.9 ± 15.5 | 0.049 |
| Velocity E (cm/s) | 61.1 ± 10.5 | 69.4 ± 16.4 | ns |
| Velocity A (cm/s) | 63.4 ±15.7 | 61.3 ± 14.5 | ns |
| E/A | 1.0 ± 0.3 | 1.2 ± 0.5 | ns |
| Velocity e’ (cm/s) | 9.6 ± 1.6 | 9.1 ± 3.2 | ns |
| E/e’ | 6.4 ± 1.0 | 6.8 ± 1.3 | ns |
| PAPs (mmHg) | 24.0 ± 6.5 | 34.1 ± 11.9 | 0.031 |
| TRV (m/s) | 1.9 ± 0.7 | 2.6 ± 0.5 | 0.046 |
| RA area (cm2) | 21.5 ± 4.9 | 21.3 ± 3.2 | ns |
| ICVd (mm) | 14.2 ± 4.1 | 18.3 ± 1.6 | 0.016 |
| TAPSE (mm) | 26.3 ± 4.2 | 27.5 ± 4.4 | ns |
Table 3: Echocardiographic parameters in mild to moderate and severe SLE patients.
Significant increases in LV end-diastolic volume (84.3 ± 15.3 ml vs. 105.9 ± 23.9 ml, p=0.028), absolute LA volume (47.5 ± 14.9 ml vs. 65.4 ± 20.1 ml, p=0.039) and indexed LA volume (28.5 ± 8.4ml/m2 vs. 39.9 ± 15.5ml/m2, p=0.049) were also determined in the severe vs. mildmoderate group. The mean LA volume of the severe group was larger than the upper value for normal adults reported in the international “Recommendations for cardiac chamber quantification” (<58 ml for males and <52 ml for females) [20]. In addition, diastolic dysfunction was detected in 9 of the 23 patients (7 with E/A <1 and 2 with E/A >2), with no significant differences between the severe and mild-moderate groups (0.0% vs. 20.0%, p=ns) (Table 3).
Right heart parameters, including pulmonary arterial pressure, TRV and IVC diameter, were significantly increased in the severe compared to the mild-moderate group. Right atrial area was larger than the upper value for normal adults [20] but not significantly different between the two groups. No significant differences were determined for any of the other parameters (Table 3).
Since the Framingham score was not significantly different between patients with severe vs. mild-moderate SLE (Table 2B), we examined the data of all 23 patients for a possible correlation between the Framingham score and echocardiographic measurements. We found that the Framingham score was directly related to LV mass, interventricular septum thickness and posterior wall diameter, as expected due to the correlation of these parameters with the blood pressure level even in normotensive patients (Figure 3).
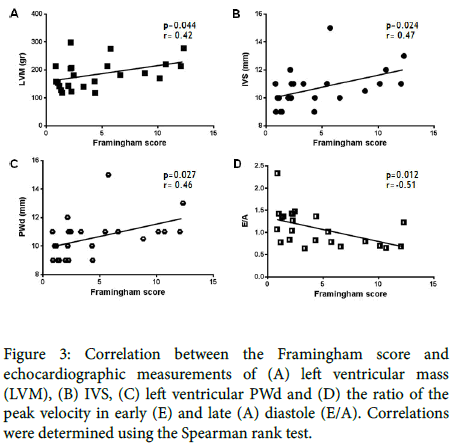
Figure 3. Correlation between the Framingham score and echocardiographic measurements of (A) left ventricular mass (LVM), (B) IVS, (C) left ventricular PWd and (D) the ratio of the peak velocity in early (E) and late (A) diastole (E/A). Correlations were determined using the Spearman rank test.
No such relationship was found for LV diameter, LV end-diastolic volume and LA volume (data not shown). However, there was an inverse correlation between the Framingham score and diastolic dysfunction (E/A) (Figure 3). None of the correlations of the other echocardiographic parameters were significant (data not shown). Finally, a direct correlation (r=0.42; p=0.049) between disease duration and echocardiographic LV end-diastolic volume values was identified (Figure 4).
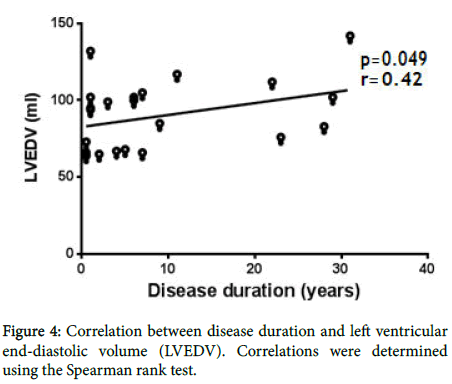
Figure 4. Correlation between disease duration and left ventricular end-diastolic volume (LVEDV). Correlations were determined using the Spearman rank test.
Cardiovascular disease has emerged as one of the most important causes of death in SLE patients [21]. Consequently, the early identification of patients at increased risk is an essential step in the successful prevention of CVD in this population. During the active phase of SLE, patients may develop subclinical myocarditis associated with enlargement of the heart chambers [22]. Here, it is important to emphasize that in this study patients with hypertension or any congenital or acquired CVD event that could have influenced the echocardiographic measurements were excluded from participation.
In the 23 patients enrolled in the study, there was no correlation between disease activity and traditional risk factors such as smoking and hyperlipidemia. With the exception of triglyceride levels, the values of all laboratory parameters were similar in patients with mildmoderate and severe SLE, thus indicating that these values cannot be used to identify patients at higher risk of CVD. The Framingham score, commonly used in the CVD risk chart, was also ineffective in predicting which patients were at the highest risk of CVD; in fact, the mean values of the two study groups were not substantially different.
We therefore used echocardiographic measurements to examine the relationship between SLE and CVD. The results of those analyses showed that absolute and indexed LV mass and LA volume as well as LV end-diastolic volume were significantly higher in patients with severe than mild-moderate SLE. While the values in the former indicated LV diastolic dysfunction, neither the E/A ratio nor the E/e’ ratio differed significantly between the two groups. The lack of alterations in the early and late diastolic velocities can be explained by the fact that the SLE patients in our study were relatively young and had stable disease, such that the elasticity reserve of the left ventricle was still intact. However, the increased LV end-diastolic volume implied myocardial involvement.
Further evidence of LV diastolic dysfunction in severe SLE came from the significant increase in LA volume determined in this group of patients. This finding is consistent with the close link between LA dynamics and LV filling pressure. During diastole, the greater LV filling pressure reflects the greater pressure gradient vs. the left atrium, which in turn is a consequence of atrial enlargement [23]. Several studies have described LV diastolic dysfunction in SLE patients, including that it is more pronounced in patients with active disease [2,14,24-32]. In our study, the Framingham score correlated directly with LV mass, LV posterior wall diameter, interventricular septum thickness and, indirectly with the E/A ratio. These results provide evidence for subclinical myocardial involvement in patients with severe SLE.
Although the precise mechanisms responsible for these cardiac abnormalities in SLE patients are unknown, coronary artery disease, caused by premature atherosclerosis, endothelial damage, immune complex-mediated inflammation, renal failure, hypertension, dyslipidemia and toxicity from medications such as GCs, cyclophosphamide and chloroquine, is likely to be involved [2,10,29]. Previous studies have reported evidence of a relationship between CVD and the exacerbation of atherogenesis by systemic inflammation [8,33,34]. In an animal model, the relationship between systemic immune activation, arterial inflammation and coronary lesions was shown to be significant. Activated immune cells infiltrate atherosclerotic plaques, where they release interleukins, tumor necrosis factor-α, platelet-derived growth factor and other cytokines that enhance the formation of atherosclerotic lesions. Atherogenesis is also supported by a cellular immune response specifically directed at oxidized low-density lipoprotein, heat-shock proteins, cardiolipin and β2-glycoprotein-I [35]. It should also be emphasized that the use of immunosuppressive therapy may not adequately target the chronic atherogenic inflammatory microenvironment [36]. An alternative explanation for the cardiac hypertrophy seen in our SLE patients is that enhanced inflammation results in salt retention, as chloride is necessary for immune cell function and sodium is a potent fluid modulator that contributes to fluid retention, thus aggravating diastolic dysfunction and myocardial hypertrophy [37-39]. This hypothesis is supported by the larger IVC diameter measured in patients severe than mild-to-moderate SLE. The higher TRV and pulmonary arterial pressure in the severe group may reflect fluid overload or pulmonary capillaritis, both of which occur in patients with SLE of longer duration [40-42].
Our study also had several limitations. First, it lacked a control group. Second, the majority of the patients were young, which might have influenced the results. Third, the exams were conducted by a single observer. Finally, perhaps the most important limitation was the small number of patients. Our findings should therefore be interpreted with caution and evaluated in further studies of larger SLE populations.
Nevertheless, our results demonstrate the utility of echocardiography as a largely available non-invasive screening tool in SLE patients. Increases in LV mass and LA volume may allow the identification of SLE patients with early-stage myocardial damage. Since diastolic dysfunction typically develops at the initial phase of LV hypertrophy and can remain asymptomatic for several years, it would be important to determine whether these abnormalities are potentially reversible after clinical remission in patients with active disease.
PL, SC, and VR planned the study and prepared the draft of the manuscript. MP contributed to the study design. NS, LC, AB, and PC cared for the patients, collected data and contributed to data analyses and interpretation. FD, AV and VR provided critical revisions of the manuscript related to its intellectual content. All authors reviewed and approved the manuscript and accept their accountability for all aspects of this study.
This work was supported by the Italian Association for Cancer Research (AIRC) through an Investigator Grant (no. 20441) to VR.
Citation: Leone P, Cicco S, Prete M, Susca N, Crudele L, Buonavoglia A, et al. (2019) Early Echocardiographic Detection of Left Ventricular Diastolic Dysfunction in Patients with Systemic Lupus Erythematosus Asymptomatic for Cardiovascular Disease . Lupus Open Access 4: 139.
Received: 19-Dec-2018 Accepted: 12-Apr-2019 Published: 19-Apr-2019
Copyright: © 2019 Leone P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.